Medicine:Endocarditis
| Endocarditis | |
|---|---|
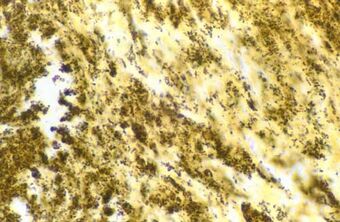 | |
| Bartonella henselae bacilli in cardiac valve of a patient with blood culture-negative endocarditis. The bacilli appear as black granulations. | |
| Specialty | Cardiology, infectious disease |
Endocarditis is an inflammation of the inner layer of the heart, the endocardium. It usually involves the heart valves. Other structures that may be involved include the interventricular septum, the chordae tendineae, the mural endocardium, or the surfaces of intracardiac devices. Endocarditis is characterized by lesions, known as vegetations, which is a mass of platelets, fibrin, microcolonies of microorganisms, and scant inflammatory cells.[1] In the subacute form of infective endocarditis, the vegetation may also include a center of granulomatous tissue, which may fibrose or calcify.[2]
There are several ways to classify endocarditis. The simplest classification is based on cause: either infective or non-infective, depending on whether a microorganism is the source of the inflammation or not. Regardless, the diagnosis of endocarditis is based on clinical features, investigations such as an echocardiogram, and blood cultures demonstrating the presence of endocarditis-causing microorganisms.
Signs and symptoms include fever, chills, sweating, malaise, weakness, anorexia, weight loss, splenomegaly, flu-like feeling, cardiac murmur, heart failure, petechia (red spots on the skin), Osler's nodes (subcutaneous nodules found on hands and feet), Janeway lesions (nodular lesions on palms and soles), and Roth's spots (retinal hemorrhages).
Infective endocarditis
Infective endocarditis is an infection of the inner surface of the heart, usually the valves.[3] Symptoms may include fever, small areas of bleeding into the skin, heart murmur, feeling tired, and low red blood cells.[3] Complications may include valvular insufficiency, heart failure, stroke, and kidney failure.[4][3]
The cause is typically a bacterial infection and less commonly a fungal infection.[3] Risk factors include valvular heart disease including rheumatic disease, congenital heart disease, artificial valves, hemodialysis, intravenous drug use, and electronic pacemakers.[5] The bacterial most commonly involved are streptococci or staphylococci.[3]
The diagnosis of infective endocarditis relies on the Duke criteria, which were originally described in 1994 and modified in 2000. Clinical features and microbiological examinations are the first steps to diagnose an infective endocarditis. The imaging is also crucial. Echocardiography is the cornerstone of imaging modality in the diagnosis of infective endocarditis. Alternative imaging modalities as computer tomography, magnetic resonance imaging, and positron emission tomography/computer tomography (PET/CT) with 2-[18F]fluorodeoxyglucose (FDG) are playing an increasing role in the diagnosis and management of infective endocarditis.[6]
The usefulness of antibiotics following dental procedures has changed over the time.[7] PRevention is recommended in patients at high risk.[3] Treatment is generally with intravenous antibiotics.[3] The choice of antibiotics is based on the blood cultures.[3] Occasionally heart surgery is required.[3][8] Populations at high risk of infective endocarditis include patients with previous infective endocarditis, patients with surgical or transcatheter prosthetic valves or post-cardiac valve repair, and patients with untreated CHD and surgically corrected congenital heart diease.[9][10]
The number of people affected is about 5 per 100,000 per year.[5] Rates, however, vary between regions of the world.[5] Males are affected more often than females.[3] The risk of death among those infected is about 25%.[5] Without treatment it is almost universally fatal.[3]
Non-infective endocarditis
Nonbacterial thrombotic endocarditis (NBTE) is most commonly found on previously undamaged valves.[2] As opposed to infective endocarditis, the vegetations in NBTE are small, sterile, and tend to aggregate along the edges of the valve or the cusps.[2] Also unlike infective endocarditis, NBTE does not cause an inflammation response from the body.[2] NBTE usually occurs during a hypercoagulable state such as system-wide bacterial infection, or pregnancy, though it is also sometimes seen in patients with venous catheters.[2] NBTE may also occur in patients with cancers, particularly mucinous adenocarcinoma[2] where Trousseau syndrome can be encountered. Typically NBTE does not cause many problems on its own, but parts of the vegetations may break off and embolize to the heart or brain, or they may serve as a focus where bacteria can lodge, thus causing infective endocarditis.[2]
Another form of sterile endocarditis is termed Libman–Sacks endocarditis; this form occurs more often in patients with lupus erythematosus and is thought to be due to the deposition of immune complexes.[2] Like NBTE, Libman-Sacks endocarditis involves small vegetations, while infective endocarditis is composed of large vegetations.[2] These immune complexes precipitate an inflammation reaction, which helps to differentiate it from NBTE. Also unlike NBTE, Libman-Sacks endocarditis does not seem to have a preferred location of deposition and may form on the undersurfaces of the valves or even on the endocardium.[2]
References
- ↑ Harrison's Principles of Internal Medicine. McGraw-Hill. May 2005. pp. 731–740. ISBN 978-0-07-139140-5. OCLC 54501403.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 Robbins Basic Pathology (8th ed.). Saunders/Elsevier. 2007. pp. 406–408. ISBN 978-1-4160-2973-1.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Infective Endocarditis - Cardiovascular Disorders" (in en-CA). September 2017. http://www.merckmanuals.com/en-ca/professional/cardiovascular-disorders/endocarditis/infective-endocarditis.
- ↑ Njuguna, B; Gardner, A; Karwa, R; Delahaye, F (February 2017). "Infective Endocarditis in Low- and Middle-Income Countries.". Cardiology Clinics 35 (1): 153–163. doi:10.1016/j.ccl.2016.08.011. PMID 27886786.
- ↑ 5.0 5.1 5.2 5.3 Ambrosioni, J; Hernandez-Meneses, M; Téllez, A; Pericàs, J; Falces, C; Tolosana, JM; Vidal, B; Almela, M et al. (May 2017). "The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century.". Current Infectious Disease Reports 19 (5): 21. doi:10.1007/s11908-017-0574-9. PMID 28401448.
- ↑ Hubers, Scott A.; DeSimone, Daniel C.; Gersh, Bernard J.; Anavekar, Nandan S. (May 2020). "Infective Endocarditis: A Contemporary Review" (in en). Mayo Clinic Proceedings 95 (5): 982–997. doi:10.1016/j.mayocp.2019.12.008. PMID 32299668.
- ↑ Cahill, TJ; Harrison, JL; Jewell, P; Onakpoya, I; Chambers, JB; Dayer, M; Lockhart, P; Roberts, N et al. (June 2017). "Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis.". Heart 103 (12): 937–944. doi:10.1136/heartjnl-2015-309102. PMID 28213367. http://eprints.whiterose.ac.uk/112532/7/Cahill_et_al_13_12_16.pdf.
- ↑ Delgado, V; Ajmone Marsan, N; de Waha, S; Bonaros, N; Brida, M; Burri, H; Caselli, S; Doenst, T et al. (14 October 2023). "2023 ESC Guidelines for the management of endocarditis.". European Heart Journal 44 (39): 3948–4042. doi:10.1093/eurheartj/ehad193. PMID 37622656.
- ↑ Delgado, V; Ajmone Marsan, N; de Waha, S; Bonaros, N; Brida, M; Burri, H; Caselli, S; Doenst, T et al. (14 October 2023). "2023 ESC Guidelines for the management of endocarditis.". European Heart Journal 44 (39): 3948–4042. doi:10.1093/eurheartj/ehad193. PMID 37622656.
- ↑ Verzelloni Sef, A; Jaggar, SI; Trkulja, V; Alonso-Gonzalez, R; Sef, D; Turina, MI (2 May 2023). "Factors associated with long-term outcomes in adult congenital heart disease patients with infective endocarditis: a 16-year tertiary single-centre experience.". European Journal of Cardio-Thoracic Surgery 63 (5). doi:10.1093/ejcts/ezad105. PMID 36946284.
Further reading
- "Cardiac conduction abnormalities in endocarditis defined by the Duke criteria". American Heart Journal 142 (2): 280–285. 2001. doi:10.1067/mhj.2001.116964. PMID 11479467.
- "Value and limitations of the von Reyn, Duke, and modified Duke criteria for the diagnosis of infective endocarditis in children". Pediatrics 112 (6 Pt 1): e467–e471. 2003. doi:10.1542/peds.112.6.e467. PMID 14654647. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=14654647.
External links
| Classification | |
|---|---|
| External resources |
 |
