Medicine:Artificial urinary sphincter
| Artificial urinary sphincter | |
|---|---|
| Script error: No such module "Image array". | |
| Other names | Inflatable artificial sphincter |
| Specialty | Urology |
| ICD-10-PCS | 0THC0LZ |
| CPT | 53445 |
An artificial urinary sphincter (AUS) is an implanted device to treat moderate to severe stress urinary incontinence, most commonly in men. The AUS is designed to supplement the function of the natural urinary sphincter that restricts urine flow out of the bladder.
Description
There are two types of artificial urinary sphincters:
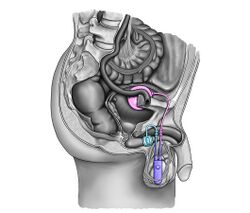
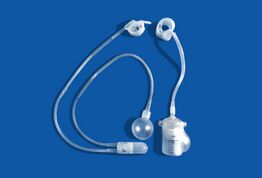
- The artificial urinary sphincter with a balloon reservoir (3-component): cuff, pump and balloon. The cuff is placed around the urethra; the pump is inserted in the scrotum and the balloon reservoir is implanted in the retropubic space – between bladder and iliac vein. The pressure in the hydraulic circuit is generated by the elastic balloon reservoir and from retropubic pressure.[1][2]
- The artificial urinary sphincter with a spring (2-component): cuff and pump unit.[3][4] The cuff is placed around the urethra and the pump unit is inserted in the scrotum. The pressure in the hydraulic circuit is generated by the spring of the pump unit. The pressure in the retropubic space does not have any influence for this type of sphincter.
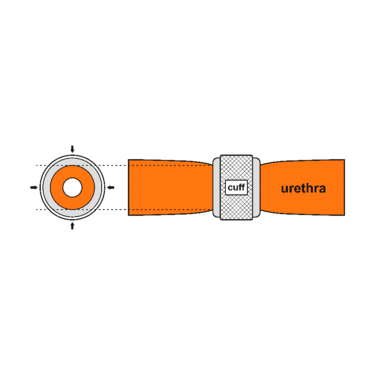
The common theme among currently available designs is a mechanical constriction mechanism – an inflatable cuff filled with sterile saline solution and placed around the urethra which keeps the urethral lumen closed; this is due to the pressure produced inside the device and an externally accessible control pump mechanism placed between two skin layers of the scrotum (subdartos pouch) which allows the user to manually relieve the constriction to allow urination.
History
Frederic Foley was the first to describe an externally worn artificial urinary sphincter to treat urinary incontinence, published in 1947.[5] In 1972, F. Brantley Scott and colleagues from Baylor College of Medicine designed the first precursor of contemporary artificial urinary sphincter.[6][7] The first AUS model on the market was the AMS 800 (Boston Scientific, Marlborough, Massachusetts ), developed 50 years ago.[8][9] It is a 3-component device with a cuff placed around the urethra, a pump inserted in the scrotum and a pressure generating reservoir placed in the pelvis, which comes as a kit to prepare and to fill up before implantation.[10]
Another AUS model is the ZSI 375 (Zephyr Surgical Implants, Geneva, Switzerland ), introduced in 2008.[11] It is a one-piece two-part device with a cuff and a pump unit with an integrated spring; it comes in one piece, pre-connected and pre-filled.[1][12] There is no abdominal component in the ZSI 375, which along with its ready-to-implant configuration reduces the operating time.[13] Furthermore, because there is no abdominal component, surgical interventions in the retroperitoneal space are not required. Previous surgeries, such as radical prostatectomy, may lead to post-operative scarring and fibrosis in the retroperitoneal space. Thus, avoiding dissection of retroperitoneal tissues avoids risks of surgical complications.[14][15] Another advantage of the ZSI 375 model is the possibility to increase or decrease the pressure inside the device after implantation to meet the desired continence rate and satisfaction of the patient. These adjustments particularly help to control continence in cases of post-implantation urethral atrophy or urinary retention (poor urine flow).[16][17][13] Adjustment of the pressure can be done in an outpatient setting by adding or removing sterile saline solution via a syringe through the scrotum.[12] By 2019, more than 4,500 ZSI 375 artificial urinary sphincters have been implanted worldwide.[11] In addition to the devices mentioned above, new devices are being brought to market, such as the Rigicon ContiClassic and ContiReflex Artificial Urinary Sphincter systems. [18][19]
In both models, sterile saline solution inside the system is used to generate pressure and compress the urethra (to prevent urine from leaking). The urethral cuff is deflated manually by pressing the control pump that is placed in the scrotum, allowing the patient to empty the bladder. The urethral cuff then re-inflates automatically to refill the urethral cuff and once again prevent urine from leaking.[20][21]
The list includes AUS models available in 2023:
| Product | Company | Country of origin | Introduced in | Design | Preconnected and prefilled | Pressure deliver | Pressure adjustable |
|---|---|---|---|---|---|---|---|
| AMS 800 | Boston Scientific (formerly American Medical Systems) | United States of America | 1988 | 3-components: cuff, pump, balloon reservoir | No | Flexible reservoir inserted in pelvic floor | No |
| ContiClassic | Rigicon Innovative Urological Solutions | United States of America | 2020 | 3-components: cuff, pump, balloon reservoir | No | Flexible reservoir inserted in pelvic floor | No |
| ContiReflex | Rigicon Innovative Urological Solutions | United States of America | 2020 | 3-components: cuff, pump, balloon reservoir | No | Flexible reservoir inserted in pelvic floor | Yes |
| ZSI 375 | Zephyr Surgical Implants | Switzerland | 2008 | 2-components: cuff, pump unit | Yes | Stainless steel spring inside the pump unit inserted in scrotum | Yes |
Medical use
The intrinsic sphincter deficiency leading to stress incontinence is the most common indication for AUS implantation.[9] The European Association of Urology recommends AUS implantation for moderate-to-severe stress incontinence in men.[22] Additionally, despite the novel treatment options (slings, urethral bulking injections, stem-cell therapy), AUS is considered to be the gold standard surgical management both for stress incontinence in men and for urinary incontinence developed as a complication of surgery, such as prostatectomy, cystectomy and TURP.[8][4][3]
There are several case reports published in the literature of AUS implantation in children for secondary incontinence resulting from traumatic urethral injury.[23][24]
There is limited data on AUS use in women, and not every product available in the market is designed for use in women.[25][26] The European Association of Urology provides limited recommendation on AUS use in women, stating that although cure is possible the risk of complication is high.[22] Nonetheless, AUS has been used as a last resort for treating urinary incontinence in women due to congenital causes and secondary to neurological diseases.[25]
Outcomes
Success rate
Numerous studies have been published regarding the outcomes of patients that have undergone artificial urinary sphincter implantation. The success rate, generally defined as achieving total (no pad use) or social continence (use of ≤1 pad/day) with the implanted device, ranges from 61% to 100% in the literature.[4] Improvement in quality of life has also been considered as success even if more than 1 pad/day was needed. The success rate was reported at 78% with a 3-year follow-up,[27] and over 72% with 5 to 7 years of follow-up.[28] In a recent systematic review, the success rate was reported to be 79% with follow-up period ranging from 5 months to 16 years.[29] A comparative study among patients implanted with different models of artificial urinary sphincter and achieved social continence showed no difference between two groups in regards of urodynamic tests, such as flow rate, urethral pressure, etc.[2] A randomised controlled trial found that the artificial urinary sphincter was non-inferior to the male or synthetic sling with respect to improving incontinence. The male sling was more cost-effective.[30][31]
Satisfaction
In different studies with a mean follow-up of more than 6 years,[32][33] at least 73% of men with an implanted artificial urinary sphincter were satisfied or very satisfied with the device, and 10-23% reported dissatisfaction. At shorter periods of follow-up (2–4 years) the satisfaction rates achieved over 90%.[29][32][4] In another study with mean follow-up of over 7 years, the overall satisfaction rate measured 3.9 on a scale from 0 to 5.[32] The satisfaction rate in patients after radiotherapy does not seem to be unfavorably affected.[34] The initial satisfaction with the continence rate is reported to be improved by adjusting the pressure inside the implant with the ZSI 375 model.[26]
Surveys of patients that underwent the procedure have found that over 90% would recommend the procedure to a friend or relative with the same problem, and over 90% would undergo the implantation again.[35][36] Along with this, 14% of patients reported improvement in sexual activity.[36]
The quality of life after AUS implantation has been shown to be significantly improved in numerous studies using various scaling tools.[29][7] And the quality of life appears not to be adversely affected by reinterventions, providing that the device continues to function after the revision.[citation needed]
Reoperation
In the largest available series evaluating 1082 patients that underwent primary AUS placement, the 5-year device survival rate was 74% which is consistent with the reported outcomes in the literature, ranging from 59% to 79%.[37] Notably, in all series, over time some patients needed to undergo a repeat surgery for recurrent urinary incontinence or infection of the device. In a pooled analysis of the available studies the reintervention rate (for any cause) was roughly 26%.[29] Significantly, some studies have demonstrated that surgeons who perform this procedure more frequently (high-volume surgeons) have improved outcomes compared to those who do them less frequently.[26] In fact, in this series the reoperative rates decreased by approximately 50% as surgeons reached their 200th case emphasizing the need for potential patients to seek high volume surgeons to improve their chance of success.[26]
Complications
Possible risks arising from the implantation of the AUS include:[1]
- injury to the urethra or bladder during AUS placement;
- difficulties emptying the bladder requiring temporary self-catheterization;
- persistent stress urinary incontinence;
- infection of the device leading to removal;
- recurrent incontinence from either device failure or atrophy of the urethral tissues (in which case further surgery can remove the old device and replace it with a new one).
The overall reported complication rate in males is 37%.[38] The most common postoperative complications are:
- mechanical failure (8-21%)
- urethral erosion (4-15%)
- infection (1-14%)
- urethral atrophy (4-10%)
Other less frequent complications are hematoma, urethral stenosis, urinary fistula.[39] Mechanical failures and non-mechanical complications may lead to surgical revision in 8-45% and 7-17% of cases, respectively. The overall device explantation rates in males is reported to be 16-20%.[40]
One of the causes of mechanical failure are the complications related to the balloon reservoir. It has been reported that 26% of men with an implanted AUS required reoperation at the 10-year follow-up, in order to regulate the pressure inside the device.
Follow-up
After discharge
Sexual intercourse should be avoided for the first 6 weeks after the procedure to allow the wound to heal properly.[41] Physical activities that put direct pressure on the wound, such as horseback and bike riding, should also be avoided for at least 6 weeks. Patients may be prescribed with a scrotal support to be worn for 1 week after the procedure.[42]
Ongoing care
To minimize the risk of damage to their AUS or urethra, it is vital that the patient informs their health care provider they have an AUS fitted before any urinary catheter placement, cystoscopy or any other medical intervention on the urinary tract. [43] Deactivating the device at nights may be recommended to patients, especially those who report being dry at night, to minimize the risks of urethral atrophy.[44][41]
Image gallery
-
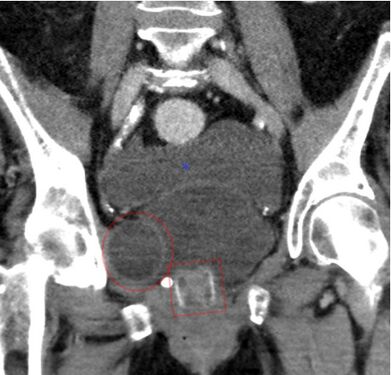
CT scan (coronal reconstruction) showing an AMS 800 in a woman
-
An X-ray image of implanted ZSI 375. The device is deactivated – the spring is compressed below the top of the cylinder. Patient is incontinent.
-
An X-ray image of implanted ZSI 375. The device is activated – the spring is decompressed at the top of the cylinder. Patient is continent.
-
An animated GIF showing how the periurethral cuff of the AUS constricts the urethra
See also
- Urinary incontinence
- Stress incontinence
- Prostatectomy
- Transurethral resection of the prostate
- Internal urethral sphincter
- Urethra
References
- ↑ 1.0 1.1 1.2 Vakalopoulos, Ioannis; Kampantais, Spyridon; Laskaridis, Leonidas; Chachopoulos, Vasileios; Koptsis, Michail; Toutziaris, Chrysovalantis (2012). "New Artificial Urinary Sphincter Devices in the Treatment of Male Iatrogenic Incontinence". Advances in Urology 2012: 1–6. doi:10.1155/2012/439372. PMID 22567002.
- ↑ 2.0 2.1 Ripert, Thomas; Pierrevelcin, Jean (February 2018). "Comparative study of urodynamic tests after AMS 800 and ZSI 375 insertion". Urologia Journal 85 (1): 15–18. doi:10.5301/uj.5000271. PMID 28967063.
- ↑ 3.0 3.1 Bauer, Ricarda M.; Gozzi, Christian; Hübner, Wilhelm; Nitti, Victor W.; Novara, Giacomo; Peterson, Andrew; Sandhu, Jaspreet S.; Stief, Christian G. (June 2011). "Contemporary Management of Postprostatectomy Incontinence". European Urology 59 (6): 985–996. doi:10.1016/j.eururo.2011.03.020. PMID 21458914.
- ↑ 4.0 4.1 4.2 4.3 Cordon, Billy H; Singla, Nirmish; Singla, Ajay K (4 July 2016). "Artificial urinary sphincters for male stress urinary incontinence: current perspectives". Medical Devices: Evidence and Research 2016 (9): 175–183. doi:10.2147/MDER.S93637. PMID 27445509.
- ↑ Foley, Frederic E.B. (October 1947). "An Artificial Sphincter: A New Device and Operation for Control of Enuresis and Urinary Incontinence". Journal of Urology 58 (4): 250–259. doi:10.1016/S0022-5347(17)69552-1. PMID 20266239. https://www.sciencedirect.com/science/article/abs/pii/S0022534717695521. Retrieved 5 February 2020.
- ↑ Scott, F. Brantley; Bradley, William E.; Timm, Gerald W. (July 1974). "Treatment of Urinary Incontinence By An Implantable Prosthetic Urinary Sphincter". Journal of Urology 112 (1): 75–80. doi:10.1016/S0022-5347(17)59647-0. PMID 4802066. https://www.sciencedirect.com/science/article/abs/pii/S0022534717596470. Retrieved 5 February 2020.
- ↑ 7.0 7.1 Yafi, Faysal A.; Powers, Mary K.; Zurawin, Jonathan; Hellstrom, Wayne J.G. (April 2016). "Contemporary Review of Artificial Urinary Sphincters for Male Stress Urinary Incontinence". Sexual Medicine Reviews 4 (2): 157–166. doi:10.1016/j.sxmr.2015.11.004. PMID 27872025.
- ↑ 8.0 8.1 Suarez, Oscar A.; McCammon, Kurt A. (June 2016). "The Artificial Urinary Sphincter in the Management of Incontinence". Urology 92: 14–19. doi:10.1016/j.urology.2016.01.016. PMID 26845050. https://www.goldjournal.net/article/S0090-4295(16)00079-0/fulltext. Retrieved 24 January 2020.
- ↑ 9.0 9.1 Scott, F. B.; Bradley, W. E.; Timm, G. W. (1974-07-01). "Treatment of urinary incontinence by an implantable prosthetic urinary sphincter". The Journal of Urology 112 (1): 75–80. doi:10.1016/s0022-5347(17)59647-0. ISSN 0022-5347. PMID 4600662.
- ↑ AMS 800™ Urinary Control System For Male Patients: Operating Room Manual. Minnetonka, MN: Boston Scientific Corporation. 2017. https://www.bostonscientific.com/content/dam/Manuals/us/current-rev-en/92116964-01A_AMS_800_ORM_en_s.pdf. Retrieved 24 January 2020.
- ↑ 11.0 11.1 Zephyr Surgical Implants (November 2019). ARTIFICIAL URINARY SPHINCTER ZSI 375 (Second ed.). Geneva, Switzerland: Zephyr Surgical Implants. https://www.zsimplants.ch/documents/zsi375/375-Patient-Selection-Manual-for-Urologists-BOOK.pdf. Retrieved 19 January 2020.
- ↑ 12.0 12.1 Ostrowski, Ireneusz; Golabek, Tomasz; Ciechan, Janusz; Śledź, Emil; Przydacz, Mikolaj; Dyś, Wojciech; Blewniewski, Mariusz; von Heyden, Burkhard et al. (2019). "Preliminary outcomes of the European multicentre experience with the ZSI 375 artificial urinary sphincter for treatment of stress urinary incontinence in men". Central European Journal of Urology 72 (3): 263–269. doi:10.5173/ceju.2019.1920. PMID 31720028.
- ↑ 13.0 13.1 Ostrowski, Ireneusz; Ciechan, Janusz; Sledz, Emil; Dys, Wojciech; Golabek, Tomasz; Chłosta, Piotr L. (2018). "Four-year follow-up on a ZSI 375 artificial urinary sphincter for male urinary incontinence from one urological centre in Poland". Central European Journal of Urology 71 (3): 320–325. doi:10.5173/ceju.2018.1704. PMID 30386654.
- ↑ Sandhu, Jaspreet S.; Maschino, Alexandra C.; Vickers, Andrew J. (December 2011). "The Surgical Learning Curve for Artificial Urinary Sphincter Procedures Compared to Typical Surgeon Experience". European Urology 60 (6): 1285–1290. doi:10.1016/j.eururo.2011.05.048. PMID 21665357. PMC 3646622. https://www.europeanurology.com/article/S0302-2838(11)00596-3/fulltext. Retrieved 27 January 2020.
- ↑ Staerman, Frederic; G-Llorens, Christophe; Leon, Priscilla; Leclerc, Yves (April 2013). "ZSI 375 artificial urinary sphincter for male urinary incontinence: a preliminary study". BJU International 111 (4b): E202–E206. doi:10.1111/j.1464-410X.2012.11468.x. PMID 22937774.
- ↑ Obando, Alejandro Carvajal; Gil, Federico Gavira; Martinez, Álvaro Gutiérrez; Molina, Luis Fernando Echeverry; Botero, Juan Carlos Castaño (1 June 2017). "Efficacy of the Artificial Urinary Sphincter ZSI 375 for Treatment of Post-Radical Prostatectomy Incontinence in Patients with Intrinsic Sphincter Deficiency: A Preliminary Study". European Medical Journal 2 (2): 22–26. doi:10.33590/emj/10313028. https://www.emjreviews.com/urology/article/efficacy-of-the-artificial-urinary-sphincter-zsi-375-for-treatment-of-post-radical-prostatectomy-incontinence-in-patients-with-intrinsic-sphincter-deficiency-a-preliminary-study/. Retrieved 27 January 2020.
- ↑ Ostrowski, Ireneusz; Blewniewski, Mariusz; Neugart, Frank; von Heyden, Burkhard; Selvaggio, Oscar; Iori, Francesco; Foley, Steeve; Arjona, Manuel Fernández et al. (1 August 2017). "Multicentre experience with ZSI 375 artificial urinary sphincter for the treatment of stress urinary incontinence in men". Urologia Journal 84 (3): 148–152. doi:10.5301/uj.5000246. PMID 28574143.
- ↑ "Surgical Management of Male Stress Incontinence: Techniques, Indications, and Pearls for Success". Res Rep Urol 15: 217–232. 2023. doi:10.2147/RRU.S395359. PMID 37366389.
- ↑ "The Asia-Pacific AMS800 artificial urinary sphincter consensus statement". Int J Urol 30 (2): 128–138. February 2023. doi:10.1111/iju.15083. PMID 36375037.
- ↑ Vakalopoulos, Ioannis; Kampantais, Spyridon; Laskaridis, Leonidas; Chachopoulos, Vasileios; Koptsis, Michail; Toutziaris, Chrysovalantis (2012). "New Artificial Urinary Sphincter Devices in the Treatment of Male Iatrogenic Incontinence". Advances in Urology 2012: 1–6. doi:10.1155/2012/439372. PMID 22567002. [verification needed]
- ↑ Chung, Eric (July 2017). "Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery". Translational Andrology and Urology 6 (Supplement 2): S112–S121. doi:10.21037/tau.2017.04.12. PMID 28791230.
- ↑ 22.0 22.1 Burkhard, F.C.; Bosch, J.L.H.R.; Cruz, F.; Lemack, G.E.; Nambiar, A.K.; Thiruchelvam, N.; Tubaro, A. (2018). EAU Guidelines on Urinary Incontinence in Adults. Arnhem, The Netherlands: European Association of Urology. ISBN 978-94-92671-01-1. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urinary-Incontinence-2018-large-text.pdf. Retrieved 24 January 2020.
- ↑ Routh, Jonathan C.; Husmann, Douglas A. (1 October 2007). "Long-term continence outcomes after immediate repair of pediatric bladder neck lacerations extending into the urethra". The Journal of Urology 178 (4S): 1816–1818. doi:10.1016/j.juro.2007.05.094. PMID 17707005.
- ↑ Kandpal, DK; Rawat, SK; Kanwar, S; Baruha, A; Chowdhary, SK (2013). "Single piece artificial urinary sphincter for secondary incontinence following successful repair of post traumatic urethral injury". Journal of Indian Association of Pediatric Surgeons 18 (4): 152–154. doi:10.4103/0971-9261.121120. PMID 24347870.
- ↑ 25.0 25.1 Islah, MAR; Cho, Sung Yong; Son, Hwancheol (April 2013). "The Current Role of the Artificial Urinary Sphincter in Male and Female Urinary Incontinence". The World Journal of Men's Health 31 (1): 21–30. doi:10.5534/wjmh.2013.31.1.21. PMID 23658862.
- ↑ 26.0 26.1 26.2 26.3 Sandhu, Jaspreet S.; Maschino, Alexandra C.; Vickers, Andrew J. (2011). "The Surgical Learning Curve for Artificial Urinary Sphincter Procedures Compared to Typical Surgeon Experience". European Urology 60 (6): 1285–1290. doi:10.1016/j.eururo.2011.05.048. PMID 21665357.
- ↑ Ostrowski, Ireneusz; Blewniewski, Mariusz; Neugart, Frank; von Heyden, Burkhard; Selvaggio, Oscar; Iori, Francesco; Foley, Steeve; Fernández Arjona, Manuel et al. (29 May 2017). "Multicentre Experience with ZSI 375 Artificial Urinary Sphincter for the Treatment of Stress Urinary Incontinence in Men". Urologia Journal 84 (3): 148–152. doi:10.5301/uj.5000246. PMID 28574143.
- ↑ Llorens, Christophe; Pottek, Tobias (18 May 2017). "Urinary Artificial Sphincter ZSI 375 for Treatment of Stress Urinary Incontinence in Men: 5 and 7 Years Follow-Up Report". Urologia Journal 84 (4): 263–266. doi:10.5301/uj.5000243. PMID 28525665.
- ↑ 29.0 29.1 29.2 29.3 Van der Aa, Frank; Drake, Marcus J.; Kasyan, George R.; Petrolekas, Andreas; Cornu, Jean-Nicolas (April 2013). "The Artificial Urinary Sphincter After a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non-neurogenic Incontinence". European Urology 63 (4): 681–689. doi:10.1016/j.eururo.2012.11.034. PMID 23219375. https://www.europeanurology.com/article/S0302-2838(12)01409-1/fulltext. Retrieved 25 January 2020.
- ↑ "Male sling is as good as more complex surgery for incontinence after prostate surgery" (in en). NIHR Evidence. 2023-05-09. doi:10.3310/nihrevidence_58018. https://evidence.nihr.ac.uk/alert/male-sling-is-as-good-as-more-complex-surgery-for-incontinence-after-prostate-surgery/.
- ↑ Constable, Lynda; Abrams, Paul; Cooper, David; Kilonzo, Mary; Cotterill, Nikki; Harding, Chris; Drake, Marcus J; Pardoe, Megan N et al. (2022-08-01). "Synthetic sling or artificial urinary sphincter for men with urodynamic stress incontinence after prostate surgery: the MASTER non-inferiority RCT" (in en). Health Technology Assessment 26 (36): 1–152. doi:10.3310/TBFZ0277. ISSN 1366-5278. PMID 35972773. PMC 9421661. https://www.journalslibrary.nihr.ac.uk/hta/TBFZ0277.
- ↑ 32.0 32.1 32.2 Montague, Drogo K. (2012). "Artificial Urinary Sphincter: Long-Term Results and Patient Satisfaction". Advances in Urology 2012 (Special Issue): 835290. doi:10.1155/2012/835290. PMID 22536227.
- ↑ Chung, Eric (17 June 2014). "A state-of-the-art review on the evolution of urinary sphincter devices for the treatment of post-prostatectomy urinary incontinence: Past, present and future innovations". Journal of Medical Engineering & Technology 38 (6): 328–332. doi:10.3109/03091902.2014.899400. PMID 24936961.
- ↑ Herschorn, Sender (17 April 2013). "The artificial urinary sphincter is the treatment of choice for post–radical prostatectomy incontinence". Canadian Urological Association Journal 2 (5): 536–9. doi:10.5489/cuaj.924. PMID 18953453.
- ↑ Viers, Boyd R.; Linder, Brian J.; Rivera, Marcelino E.; Rangel, Laureano J.; Ziegelmann, Matthew J.; Elliott, Daniel S. (2016). "Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence". The Journal of Urology 196 (3): 838–843. doi:10.1016/j.juro.2016.03.076. PMID 26997310.
- ↑ 36.0 36.1 Litwiller, Scott E.; Kim, Kap B.; Fone, Patricia D.; deVere White, Ralph W.; Stone, Anthony R. (December 1996). "Post-Prostatectomy incontinence and the Artificial Urinary Sphincter: A Long-Term Study of Patient Satisfaction and Criteria for Success". Journal of Urology 156 (6): 1975–1980. doi:10.1016/S0022-5347(01)65408-9. PMID 8911369. https://www.auajournals.org/article/S0022-5347(01)65408-9/pdf. Retrieved 2 February 2020.
- ↑ Linder, Brian J.; Rivera, Marcelino E.; Ziegelmann, Matthew J.; Elliott, Daniel S. (2015). "Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic". Urology 86 (3): 602–607. doi:10.1016/j.urology.2015.05.029. PMID 26135815.
- ↑ Hussain, Mahreen; Greenwell, Tamsin J.; Venn, Suzie N.; Mundy, Anthony R. (1 August 2005). "The current role of the artificial urinary sphincter for the treatment of urinary incontinence". Journal of Urology 174 (2): 418–424. doi:10.1097/01.ju.0000165345.11199.98. PMID 23658862.
- ↑ Islah, MAR; Cho, Sung Yong; Son, Hwancheol (April 2013). "The Current Role of the Artificial Urinary Sphincter in Male and Female Urinary Incontinence". The World Journal of Men's Health 31 (2): 21–30. doi:10.5534/wjmh.2013.31.1.21. PMID 23658862.
- ↑ Amend, Bastian; Toomey, Patricia; Sievert, Karl-Dietrich (November 2013). "Artificial sphincter". Current Opinion in Urology 23 (6): 520–527. doi:10.1097/01.MOU.0000434591.02823.d0. PMID 24080811. https://new.hindawi.com/journals/au/2012/835290/. Retrieved 20 January 2020.
- ↑ 41.0 41.1 "Urinary Sphincter Replacement (Discharge Care) - What You Need to Know" (in en). https://www.drugs.com/cg/urinary-sphincter-replacement-discharge-care.html. Retrieved 25 March 2020.
- ↑ "About Your Artificial Urinary Sphincter | Memorial Sloan Kettering Cancer Center" (in en). Memorial Sloan Kettering Cancer Center. https://www.mskcc.org/cancer-care/patient-education/artificial-urinary-sphincter. Retrieved 25 March 2020.
- ↑ Agarwal, Deepak K; Linder, Brian J; Elliott, Daniel S (2016). "Artificial urinary sphincter urethral erosions: Temporal patterns, management, and incidence of preventable erosions". Indian Journal of Urology 33 (1): 26–29. doi:10.4103/0970-1591.195758. PMID 28197026.
- ↑ Elliott, Daniel S; Barrett, David M; Gohma, Mohamed; Boone, Timothy B (June 2001). "Does nocturnal deactivation of the artificial urinary sphincter lessen the risk of urethral atrophy?". Urology 57 (6): 1051–1054. doi:10.1016/s0090-4295(01)00963-3. PMID 11377302.
External links
 |