Medicine:Bioresorbable stent
| Bioresorbable stent | |
|---|---|
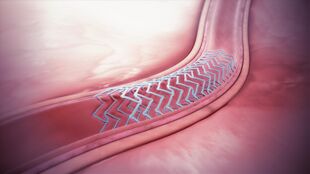 A bioresorbable stent implanted in the blood vessel. | |
| Specialty | Vascular system |
A bioresorbable stent is a tube-like device (stent) that is used to open and widen clogged heart arteries and then dissolves or is absorbed by the body. It is made from a material that can release a drug to prevent scar tissue growth. It can also restore normal vessel function and avoid long-term complications of metal stents.[1][2]
In medicine, a stent is any device which is inserted into a blood vessel or other anatomical internal duct to expand it to prevent or alleviate a blockage. Traditionally, such devices are fabricated from metal mesh and remain in the body permanently or until removed through further surgical intervention. A bioresorbable stent (also called bioresorbable scaffold, biodegradable stent or naturally-dissolving stent) serves the same purpose, but is manufactured from a material that may dissolve or be absorbed in the body.[3]
Background
The use of metal drug-eluting stents presents some potential drawbacks. These include a predisposition to late stent thrombosis, prevention of late vessel adaptive or expansive remodeling, hindrance of surgical revascularization, and impairment of imaging with multislice CT.[4][5]
To overcome some of these potential drawbacks, several companies are pursuing the development of bioresorbable scaffolds or bioabsorbable stents. Like metal stents, placement of a bioresorbable stent will restore blood flow and support the vessel through the healing process. However, in the case of a bioresorbable stent, the stent will gradually resorb and be benignly cleared from the body, enabling a natural reconstruction of the arterial wall and restoration of vascular function.[6]
Studies have shown that the most critical period of vessel healing is largely complete by approximately three to nine months.[6][7][8] Therefore, the goal of a bioresorbable or "temporary" stent is to fully support the vessel during this critical period, and then resorb from the body when it is no longer needed.
Base materials
Bioabsorbable scaffolds, or naturally dissolving stents, that have been investigated include base materials that are either metals or polymers. While polymer-based scaffolds had a strong presence at first, they have meanwhile lost some appeal due to safety concerns and focus is now moved towards metallic magnesium-based scaffolds.[9]
Metal based
Metal stent candidates are magnesium, iron, zinc and their alloys.[10]
Magnesium-based scaffolds have been approved for use in several countries around the world. The only commercially available magnesium-based scaffold consists of a magnesium alloy, approximately 95% of which resorbs within one year of implantation.[11][12][13] Thousands of commercially available magnesium-based scaffolds have been implanted. Clinical results suggest that magnesium-based scaffolds may be a viable option in avoiding the drawbacks of permanent stents.[14][15][16][17] While degrading harmlessly, it has been shown to possess a functional degradation time of about 30 days in vivo. This is much short of the three-to-six month window desired for bioabsorbable stents. Thus, much attention has been given to drastically reducing the rate of magnesium corrosion by alloying, coating, etc.[18] Many novel methods have surfaced to minimize the penetration rate and hydrogen evolution rate (or, in layman's terms, the corrosion rate). One of the most successful has involved the creation of bioabsorbable metallic glasses via rapid solidification. Other, alternative solutions have included the development of magnesium–rare-earth (Mg-RE) alloys, which benefit from the low cytotoxicity of RE elements. Coatings and sophisticated materials processing routes are currently being developed to further decrease the corrosion rate. However, a number of issues remain limiting the further development of Mg biomaterials in general.[19]
Iron stents were shown using an in vivo evaluation method based on the murine abdominal aorta to generate an iron oxide-filled cavity in the vascular wall[20] that is unlikely to be metabolized safely.[21]
Zinc shows desirable physiological corrosion behavior, meeting a benchmark penetration rate of 20 micrometers per year.[22] However, Zn has poor mechanical behavior, with a tensile strength of around 100–150 MPa and an elongation of 0.3–2%, which is far from reaching the strength required as an orthopedic implant or stent material.[23]
Polymer-based
Polymer-based stents have been approved for use in some countries around the world. These are based on poly(L-lactide) (PLLA), chosen because it is able to maintain a radially strong scaffold that breaks down over time into lactic acid, a naturally occurring molecule that the body can use for metabolism. Other polymers in development include tyrosine poly carbonate and salicylic acid.[24]
An example of a naturally dissolving stent is the 'Absorb' stent 'produced by Abbott[25] that has several design components and features: base scaffold: a poly(L-lactide) polymer similar to that in dissolvable stitches is shaped into a tube made up of zigzag hoops linked together by bridges; drug-eluting layer': a mixture of poly-D, L-lactide (PDLLA) and everolimus; 'markers': a pair of radio-opaque platinum markers at the ends that allow the device to be visualized during angiography; 'delivery system': a balloon delivery system. Recently however, Polymer-based scaffolds, in particular Poly-L-Lactide Acid (PLLA) scaffolds, have raised serious concerns on the scaffold performance particularly in terms of safety which led to the commercial discontinuation of the main representative Absorb.[26][27]
Clinical research
Clinical research has shown that resorbable scaffolds, or naturally dissolving stents, offer comparable efficacy and safety profile to drug-eluting stents. Specifically, the Magmaris resorbable magnesium scaffold[28] has reported a favorable safety profile with low target lesion failure and scaffold thrombosis rates. These clinical results are comparable to thin-strutted drug-eluting stents in similar patient populations.[29][30][31][32]
The Absorb naturally dissolving stent has also been investigated in single-arm trials and in randomized trials comparing it to a drug-eluting stent. Early and late major adverse cardiac events, revascularizations, and scaffold thromboses have been uncommon and similar to the Xience DES, a market leader in the drug eluting stent category.[33][34][35][36][37] Studies in real-world patients are ongoing.[37]
Imaging studies show that the Absorb naturally dissolving stent begins to dissolve from six to 12 months and is fully dissolved between two and three years after it is placed in the artery.[35] Two small platinum markers remain to mark the location of the original PCI. The artery is able to dilate and contract, called vasomotion, similar to a healthy blood vessel at two years.[34]
History
In the US, the first fully absorbable stent was approved by FDA in 2016.[1]
See also
References
- ↑ 1.0 1.1 "FDA approves first absorbable stent for coronary artery disease". 24 March 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-absorbable-stent-coronary-artery-disease.
- ↑ Ni, Le; Chen, Hao; Luo, Zhurong; Yu, Yunqiang (2020). "Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis". Journal of Cardiothoracic Surgery 15 (1): 26. doi:10.1186/s13019-020-1041-5. PMID 31992360.
- ↑ Zong, Jiabin; He, Quanwei; Liu, Yuxiao; Qiu, Min; Wu, Jiehong; Hu, Bo (2022-07-19). "Advances in the development of biodegradable coronary stents: A translational perspective". Materials Today Bio 16. doi:10.1016/j.mtbio.2022.100368. ISSN 2590-0064. PMID 35937578.
- ↑ Serruys, PW et al. (14 March 2009). "A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods". Lancet 373 (9667): 897–910. doi:10.1016/S0140-6736(09)60325-1. PMID 19286089.
- ↑ Ormiston, JA et al. (15 March 2008). "A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial". Lancet 371 (9616): 899–907. doi:10.1016/S0140-6736(08)60415-8. PMID 18342684.
- ↑ 6.0 6.1 Williams, PD; Awan, M (2017). "Stent selection for percutaneous coronary intervention". Continuing Cardiology Education 3 (2): 64–69. doi:10.1002/cce2.54.
- ↑ Serruys, PW et al. (February 1988). "Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months.". Circulation 77 (2): 361–71. doi:10.1161/01.CIR.77.2.361. PMID 2962786. http://repub.eur.nl/pub/4272.
- ↑ Post, MJ; Borst C; Kuntz RE (1994). "The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty: a study in the normal rabbit and the hypercholesterolemic Yucatan micropig". Circulation 89 (6): 2816–2821. doi:10.1161/01.CIR.89.6.2816. PMID 8205696.
- ↑ Husten, Larry. "Abbott Pulls Troubled Absorb Stent From European Market". CardioBrief. http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/.
- ↑ Biodegradable Metal Stents: A Focused Review on Materials and Clinical Studies. A.Purnama, H.Hermawan, and D.Mantovani. Journal of Biomaterials and Tissue Engineering Vol. 4, 1–6, 2014 [1]
- ↑ Joner, M; Ruppelt, P; Zumstein, P (2018). "Preclinical Evaluation of Degradation Kinetics and Elemental Mapping of First and Second Generation Bioresorbable Magnesium Scaffolds". EuroIntervention 2 (9): e1040–e1048. doi:10.4244/EIJ-D-17-00708. PMID 29469029.
- ↑ Haude, M; Erbel, R; Erne (2016). "Safety and performance of the Drug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicenter, first-in-man BIOSOLVE-I trial.". EuroIntervention 12 (2): e160-6. doi:10.4244/EIJ-D-15-00371. PMID 27290675.
- ↑ Kirkland, N; Birbilis N (2013). Magnesium Biomaterials: Design, Testing and Best Practice. New York: Springer. ISBN 978-3-319-02123-2. https://www.springer.com/materials/biomaterials/book/978-3-319-02122-5.
- ↑ Kang-Yin Lee, M (Sep 23, 2018). "Twelve-Month Outcomes with a Resorbable Magnesium Scaffold in a Real-world Setting". ClinicalTrials.gov: NCT02817802 (n=2054; first 400 patients presented). https://www.tctmd.com/slide/biosolve-iv-twelve-month-outcomes-resorbable-magnesium-scaffold-real-world-setting.
- ↑ Haude, M (September 22, 2018). "Imaging and Clinical Results with the latest Magmaris Magnesium-Based Scaffold". Presented at TCT.
- ↑ Haude, M; Ince, H; Abizaid, A (May 23, 2018). "Long-term clinical data and multimodality imaging analysis of the BIOSOLVE-II study with the drug-eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries – BIOSOLVE-II". Presented at EuroPCR.
- ↑ Haude, M; Erbel, R; Erne (2016). "Safety and performance of the Drug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicenter, first-in-man BIOSOLVE-I trial". EuroIntervention 12 (2): e160-6. doi:10.4244/EIJ-D-15-00371. PMID 27290675.
- ↑ Li, N; Zheng Y (2013). "Novel magnesium alloys developed for biomedical application: a review". Journal of Materials Science & Technology. ISBN 978-3-319-02123-2.
- ↑ Kirkland, Nicholas T. (2012). "Magnesium biomaterials: past, present and future". Corrosion Engineering, Science and Technology 47 (5): 322–328. doi:10.1179/1743278212Y.0000000034.
- ↑ Pierson, D; Edick J; Tauscher A; Pokorney E; Bowen PK; Gelbaugh JA; Stinson J; Getty H et al. (January 2012). "A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials". J Biomed Mater Res B 100B (1): 58–67. doi:10.1002/jbm.b.31922. PMID 21905215.
- ↑ Aljihmani, Lilia; Alic, Lejla; Boudjemline, Younes; Hijazi, Ziyad M.; Mansoor, Bilal; Serpedin, Erchin; Qaraqe, Khalid (2019). "Magnesium-Based Bioresorbable Stent Materials: Review of Reviews". Journal of Bio- and Tribo-Corrosion 5 (1). doi:10.1007/s40735-019-0216-x. ISSN 2198-4220. Bibcode: 2019JBTC....5...26A.
- ↑ Bowen, PK; Drelich J; Goldman J (14 March 2013). "Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents". Advanced Materials 25 (18): 2577–82. doi:10.1002/adma.201300226. PMID 23495090. Bibcode: 2013AdM....25.2577B. https://www.scribd.com/doc/130468782/Zinc-Exhibits-Ideal-Physiological-Corrosion-Behavior-for-Bioabsorbable-Stents. Retrieved 15 March 2013.
- ↑ Kong L, Heydari Z, Lami GH, Saberi A, Baltatu MS, Vizureanu P. A comprehensive review of the current research status of biodegradable zinc alloys and composites for biomedical applications. Materials. 2023 Jul 3;16(13):4797.https://www.mdpi.com/1996-1944/16/13/4797
- ↑ "The ABSORB bioresorbable vascular scaffold: an evolution or revolution in interventional cardiology?". Hellenic J Cardiol. 53 (4): 301–309. 2012. 22796817. PMID 22796817. http://www.hellenicjcardiol.org/archive/full_text/2012/4/2012_4_301.pdf.
- ↑ "FDA approves Abbott's Absorb™ bioresorbable stent, the only fully dissolving heart stent" (in en-us). https://abbott.mediaroom.com/2016-07-05-FDA-approves-Abbotts-Absorb-bioresorbable-stent-the-only-fully-dissolving-heart-stent.
- ↑ Montone, RA; Niccoli, G; De Marco, F; Minelli, S; D'Ascenzo, F; Testa, L; Bedogni, F; Crea, F (2017). "Temporal trends in adverse events after everolimus-eluting bioresorbable vascular scaffold versus everolimus-eluting metallic stent implantation: A meta-analysis of randomized controlled trials". Circulation 135 (22): 2145–2154. doi:10.1161/CIRCULATIONAHA.117.028479. PMID 28559495.
- ↑ Sorrentino, S; Giustino, G; Mehran, R; Kini, AS; Sharma, SK; Faggioni, M; Farhan, S; Vogel, B et al. (2017). "Everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents". J Am Coll Cardiol 69 (25): 3055–3066. doi:10.1016/j.jacc.2017.04.011. PMID 28412389.
- ↑ Galli, Stefano; Testa, Luca; Montorsi, Piero; Bedogni, Francesco; Pisano, Francesco; Palloshi, Altin; Mauro, Ciro; Contarini, Marco et al. (2022). "SICI-GISE Position Document on the Use of the Magmaris Resorbable Magnesium Scaffold in Clinical Practice". Cardiovascular Revascularization Medicine: Including Molecular Interventions 34: 11–16. doi:10.1016/j.carrev.2021.02.003. ISSN 1878-0938. PMID 33674219.
- ↑ Meredith, IExpression error: Unrecognized word "et". (2013). "Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent". EuroIntervention 9 (3): 308–15. doi:10.4244/EIJV9I3A52. PMID 23872647.
- ↑ Stone, G (Oct 22–26, 2012). "Everolimus-Eluting Stents: SPIRIT and PLATINUM Update". ClinicalTrials.gov: NCT00180310 .NCT00180479, NCT00307047. https://docplayer.net/94030160-Tct2012-program-october-22-26-miami-beach-convention-center-miami-fl.html.
- ↑ Haude, MExpression error: Unrecognized word "et". (May 23, 2018). "Long-term clinical data and multimodality imaging analysis of the BIOSOLVE-II study with the drug-eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries – BIOSOLVE-II". Presented at EuroPCR.
- ↑ Haude, M; Ince, H; Kische, S (2017). "Safety and Clinical Performance of the Drug Eluting Absorbable Metal Scaffold in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries at 12-month follow-up- BIOSOLVE-II and BIOSOLVE-III.". Journal of the American College of Cardiology 70 (18): B6–B7. doi:10.1016/j.jacc.2017.09.071.
- ↑ Ormiston JAExpression error: Unrecognized word "et". (2008). "A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial". Lancet 371 (9616): 899–907. doi:10.1016/S0140-6736(08)60415-8. 18342684. PMID 18342684.
- ↑ 34.0 34.1 Serruys, PWExpression error: Unrecognized word "et". (2009). "A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods". Lancet 373 (9667): 897–910. doi:10.1016/S0140-6736(09)60325-1. PMID 19286089.
- ↑ 35.0 35.1 Serruys, PWExpression error: Unrecognized word "et". (2014). "Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months". EuroIntervention 9 (11): 1271–1284. doi:10.4244/EIJV9I11A217. PMID 24291783. https://ruj.uj.edu.pl/xmlui/handle/item/109186.
- ↑ Serruys PWExpression error: Unrecognized word "et". (2015). "A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial". Lancet 385 (9962): 43–54. doi:10.1016/S0140-6736(14)61455-0. PMID 25230593.
- ↑ 37.0 37.1 Smits P, Ziekenhuis M, Absorb Extend: an interim report on the 36-month clinical outcomes from the first 250 patients enrolled. Presented at Transcatheter Cardiovascular Therapeutics (TCT) conference 2014 in Washington, DC, September 2014
 |
