Medicine:Drug-eluting stent
| Drug-eluting stent | |
|---|---|
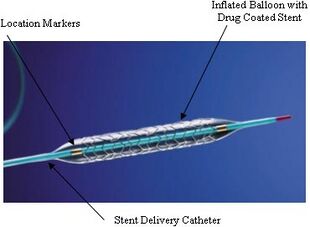 An example of a drug-eluting stent. This is the TAXUS Express2 Paclitaxel-Eluting Coronary Stent System, which releases paclitaxel. The system consists of a catheter delivery element, an inflation system, and the drug-eluting stent itself. They are marketed as one integrated system. | |
| ICD-9-CM | 00.55 |
| MeSH | D054855 |
A drug-eluting stent (DES) is a tube made of a mesh-like material used to treat narrowed arteries in medical procedures both mechanically (by providing a supporting scaffold inside the artery) and pharmacologically (by slowly releasing a pharmaceutical compound). A DES is inserted into a narrowed artery using a delivery catheter usually inserted through a larger artery in the groin or wrist. The stent assembly has the DES mechanism attached towards the front of the stent, and usually is composed of the collapsed stent over a collapsed polymeric balloon mechanism, the balloon mechanism is inflated and used to expand the meshed stent once in position. The stent expands, embedding into the occluded artery wall, keeping the artery open, thereby improving blood flow. The mesh design allows for stent expansion and also for new healthy vessel endothelial cells to grow through and around it, securing it in place.[1][2][3]
A DES is different from other types of stents in that it has a coating that delivers medication directly into the blood vessel wall. The stent slowly releases a drug to prevent the growth of scar tissue and new obstructive plaque material which caused the original blood vessel stenosis, this clogging of a stent is termed restenosis. A DES is fully integrated with a catheter delivery system and is viewed as one integrated medical device.[4][5][6]
DESs are commonly used in the treatment of narrowed arteries in the heart (coronary artery disease), but also elsewhere in the body, especially the legs (peripheral artery disease).[7] Over the last three decades, coronary stenting has matured into a primary minimally invasive treatment tool in managing CAD.[8] Coronary artery stenting is inherently tied to percutaneous coronary intervention (PCI) procedures. PCI is a minimally invasive procedure performed via a catheter (not by open-chest surgery), it is the medical procedure used to place a DES in narrowed coronary arteries. PCI procedures are performed by an interventional cardiologist using fluoroscopic imaging techniques to see the location of the required DES placement. PCI uses larger peripheral arteries in the arms or the legs to thread a catheter/DES device through the arterial system and place the DES in the narrowed coronary artery or arteries.[7] Multiple stents are often used depending on the degree of blockage and the number of diseased coronary arteries that are being treated.[9][10]
Design
A drug-eluting stent (DES) is a small mesh tube that is placed in the arteries to keep them open in the treatment of vascular disease. The stent slowly releases a drug to block cell proliferation (a biological process of cell growth and division), thus preventing the arterial narrowing (stenosis) that can occur after stent implantation. While such stents can be used in various arteries throughout the body, they are commonly placed in the coronary arteries to treat coronary heart disease.[11][12][13][14] DES products are integrated medical devices and are part of a percutaneous coronary intervention (PCI) delivery system.[15][16][17][18]
DES is a medical device with several key properties: it functions as a structural scaffold, physically keeping an artery open to ensure blood flow; the device has specific drug delivery features, and the chosen drug is critical for its effectiveness. The drug, the hallmark compenent of the device, is selected for its suitability in inhibiting restenosis and its pharmacokinetics. Apart from the drug, the materials used in the fabrication of the device are also essential and are carefully chosen for their biocompatibility and durability in a biological environment, such as human blood; these materials must also withstand the constant motion of the heart's beat and be suitable for future patient imaging using magnetic resonance imaging (MRI) technologies, which employ high magnetic fields.[15][16][17][18]
Other components, such as the catheter design, also play significant roles in the device's overall functionality and effectiveness.[15][16][17][18]
DES are typically composed of metal alloys, most commonly stainless steel or cobalt-chromium, but can also be made of other materials such as platinum-chromium or nickel-titanium. The stent is often coated with a polymer to control the release of drugs. The role of polymers in drug delivery is significant as they regulate the rate at which the drug is released into the surrounding tissue.[19][20] There are also polymer-free stents where the drug is directly coated on the stent or contained in reservoirs within the stent.[21][22][23][24]
The design of the stent includes struts, which are thin wire structures that make up the stent frame. The strut thickness can influence the stent's performance, with thinner struts generally being associated with lower restenosis rates and reduced thrombosis risk.[25][26][20]
Most DES are balloon-expandable, meaning they are mounted on a balloon catheter and expand when the balloon is inflated.[27][28] There are also self-expanding stents, which automatically expand when deployed. The very first stent, introduced in 1986, was of this type.[29][30][31][32]
The stent tube mesh is initially collapsed onto the catheter—in this collapsed state, it is small enough to be passed though relatively narrow arteries and then expanded in its destination place, pushing firmly to the diseased artery wall.[33][34]
The pharmaceutical compounds that DES emit are antiproliferative agents such as sirolimus, everolimus, zotarolimus, paclitaxel and biolimus. These drugs help prevent the arterial narrowing that can occur after stent implantation.[35][36][37] These drugs are also used for other purposes, that involve moderating the immune system or treating cancer. They work by inhibiting cell growth. In DES, they are used in very small amounts and for a short time, and only in the area where the stent is placed.[38]
There is a distinction between coronary stents and peripheral stents.[7] While both are used to prevent the narrowing of arteries, coronary stents are specifically for the coronary arteries, while peripheral stents are for any other arteries in the body.[39][40][41] Peripheral stents are mostly bare metal ones; some peripheral DES, of the self-expanding type, are used in arteries of the legs.[42]
Bioresorbable DES are made of materials that can be absorbed by the body over time, potentially reducing potential long-term complications associated with permanent stents.[43]
Uses
Atherosclerosis: a general background
Atherosclerosis is a chronic disease that affects the large and medium-sized arteries. It is characterized by the accumulation of calcium, fats (such as cholesterol) and other substances in the innermost layer of the endothelium, a layer of cells that line the interior surface of blood vessels. Atherosclerosis is considered to be the most common form of arteriosclerosis, which refers to the loss of arterial elasticity caused by thickening and stiffening of blood vessels.[44]
Atherosclerosis can begin as early as childhood with the development of small "fatty streaks" within arteries. These streaks are essentially deposits of fat. Over time, these initial lesions grow larger and become thicker, forming atheromas (atherosclerotic plaques).[44]
Drug-eluting stents (DESs) are used in the treatment of atherosclerosis in both coronary interventions and peripheral arterial interventions:[45][46]
- In coronary interventions, DESs are used to treat coronary artery disease, which is primarily caused by atherosclerosis.[45] The stents are inserted into narrowed coronary arteries and then expanded to open up the narrowed artery. The drug compound released by the stents suppresses cellular growth in the newly stented area, reducing the potential for blockage within the stent area itself.[45][47][48]
- In peripheral arterial interventions, DESs have established themselves as the go-to choice for addressing symptomatic peripheral arterial disease (PAD).[49][50][51][52][53] These highly effective stents are deployed in the treatment of peripheral arterial occlusive disease (PAOD), a condition that shares resemblances with coronary artery disease but specifically affects the peripheral arteries.[7] By employing DESs, healthcare professionals can provide optimal care and intervention to manage PAOD, ultimately improving patient outcomes and mitigating associated complications.[54]
DESs are used in the management of atherosclerosis in both coronary and peripheral arterial interventions.[7] They help improve blood flow and reduce the risk of restenosis, thereby improving patient outcomes. The use of DESs is accompanied by appropriate medical therapy and lifestyle modifications to manage atherosclerosis effectively.[51]
Stenosis and restenosis of blood vessels
Stenosis of blood vessels refers to the narrowing of the blood vessels, which can restrict blood flow to the organs and tissues.[20] This condition is often caused by the buildup of fatty deposits in the arteries, a process also called atherosclerosis.[55]
In the context of stents, stenosis is a significant concern. Stents are inserted into a narrowed artery during a procedure known as angioplasty. The stents help to open up the narrowed artery and improve blood flow. However, over time, the treated artery can close up again, a condition known as restenosis.[20]
Restenosis, or in-stent restenosis, is a blockage or narrowing that comes back in the portion of the artery previously treated with a stent.[20] Restenosis tends to happen three to six months after the procedure.[20] Restenosis is even more likely to occur if a stent would not have been used.[20]
When restenosis occurs, another procedure may be needed to correct the problem, such as the placement of a DES[20][55] that gradually release a drug compound that suppresses cellular growth, thereby reducing the potential for blockage within the stent area itself.[20][55] This therapy significantly reduces the occurrence of adverse events post-stenting.[20][55]
Technically, a DES in a mesh tube implant devices that is used in angioplasty procedures to treat stenosis of blood vessels and prevent restinosis: the stent, which elutes drugs, is implanted into the blood vessel to help keep the vessel open and improve blood flow.[56][57][58] Specifically, drug-eluting stents are used in the treatment of various medical conditions usually at the site of stenotic or occlusive arterial lesions, but one of the primary medical uses is in the treatment of coronary artery disease.[59] Stents are inserted into narrowed coronary arteries, where the narrowing is primarily caused by atherosclerosis. Stents are then expanded to open up the narrowed artery. Such stents gradually release a drug compound that suppresses cellular growth, into the newly stented area, thereby reducing the potential for blockage within the stent area itself.[59] Such blockage is termed in-stent restenosis (ISR). This in-stent blockage is most often caused by excessive cell proliferation or thrombi (blood clots). Anticoagulation therapy (blood thinners), has become a standard treatment following the placement of DES. This therapy significantly reduces the occurrence of adverse events post-stenting.[60][61][62]
Coronary interventions
DESs have played a transformative role in the management of coronary artery disease. These stents are tiny, flexible mesh tubes employed during percutaneous coronary intervention (PCI) to address narrowed coronary arteries. What sets them apart is their special coating, which incorporates a drug delivery system that enables controlled release of medication over a specific period, typically within the first 30 to 45 days following implantation. This medication aids in inhibiting the formation of scar tissue within the stent and subsequent re-narrowing of the blood vessel.[63][64]
PCI is a minimally invasive procedure. It involves the placement of a drug-eluting stent (DES) in a coronary artery. This procedure, previously known as angioplasty with a stent, is considered non-surgical as it is performed through a small puncture in a peripheral artery, avoiding the need to open the chest wall. While bleeding from the puncture site was once a concern, advancements in PCI practices have mitigated this issue through the use of pressure bands and arterial closure systems. Modern DES/PCI procedures are generally painless, although some mild discomfort may be experienced.[65][66][67] In PCI, multiple DES are sometimes implanted within a single patient; the decision to use multiple stents is typically contingent on the extent of the coronary artery disease present and the number of diseased coronary arteries that require treatment.[9][10]
Peripheral arterial interventions
DESs have emerged as the primary therapeutic approach for managing symptomatic peripheral arterial disease (PAD). These specialized stents are now widely utilized in the treatment of peripheral arterial occlusive disease (PAOD), a condition that shares similarities with coronary artery disease but affects the peripheral arteries. By deploying DESs, healthcare professionals can effectively address and alleviate the complications associated with PAOD, enhancing patient outcomes and quality of life.[68][69][51] The use of DESs in peripheral arterial interventions has shown encouraging results in terms of primary patency (PP) and target lesion revascularization (TLR) compared with bare-metal stents (BMSs).[70][71][52][53]
Different types of DESs are available on the market, each with different concentrations of drugs and showing varying efficacy.[52][53] Among the different DESs, sirolimus-eluting stents and everolimus-eluting stents were found to be more effective than paclitaxel-eluting stents.[52][53]
Clinical indications
| Coronary arteries providing blood to the heart. The blood vessels originate from the aorta and surround the heart. | |
|---|---|
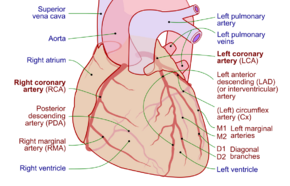 Showing the coronary arteries that are subject to narrowing - resulting in reduced blood supply to the cardiac muscle. | |
| Anatomical terminology |
PCI and stent placement are considered when someone shows signs of reduced blood flow in the arteries that supply the heart or when tests, such as different types of coronary artery imaging, show a blockage in those arteries.[72][73]
Symptoms can include:
- severe, pressure-like chest pain unrelieved by rest;
- shortness of breath, fatigue, lightheadedness;
- palpitations;
- atypical symptoms: nausea, vomiting, indigestion, confusion, back pain.[74]
In a medical setting, it's not very useful for doctors to rely solely on what people say about where their pain comes from or how it feels, because the way people describe chest pain caused by reduced blood flow to the heart can vary greatly and may not match what is typically taught in medical education or described in books and articles.[75][76]
Contraindications
DES is not recommended in some cases as it may do more harm than good. DES is not suitable:
- when individuals have a bleeding tendency;[77]
- when a coronary artery has no clear and identifiable narrowing;[78]
- when only one diseased coronary artery supplies oxygenated blood to the heart muscle. During stent placement, there is a short period of blood flow blockage by the balloon inflation. This blockage time is often longer than twenty seconds to allow the DES to expand and embed into the arterial wall. In this case, this time may be too long and cause serious events due to lack of blood to the heart muscle.[79]
Bleeding disorders make DES unsuitable because of the need for anticoagulation drugs (blood thinners) during the procedure and in post-stenting aftercare. Other factors that could rule out the use of stents include a history of in-stent blockage, bleeding problems, complex or unsuitable coronary anatomy, or a short life expectancy due to other serious medical conditions.[80]
Risks and complications
Risks from the procedure
Stent placement risks include bleeding, allergic reactions to the contrast agents used to visualize the coronary arteries, and myocardial infarction. With percutaneous coronary intervention (PCI), the requirement for emergency coronary artery bypass graft (CABG) surgery has decreased as better practices have been introduced.[81] In some situations, coronary stenting is permitted in hospitals without cardiac surgery facilities,[82] but such permission remains controversial because of the rare but unpredictable risk of coronary artery perforation.[83]
Stent thrombosis risks
A complication of coronary stenting is stent thrombosis (blood clots). This occurs when a new clot forms within the stent and occludes blood flow, causing a heart attack.[84][85][86]
In-stent restenosis risks (ISR)
DES were designed to specifically combat issues of restenosis that occurred with older bare-metal stents (BMS).[70][87] Though less frequent with drug-eluting stents, restenosis can still occur.[88]
Since the advent of DES technology, the incidence of ISR has significantly decreased.[89][90]
Usage outside the scope of typical regulatory approval
DES have been shown to be superior to BMS in reducing short-term complications of stenting in saphenous vein grafts.[91] However, the use of DESs in bypass grafts was not their originally intended use nor within the scope of originally regulatory approval (US FDA, European Medicines Agency, etc.). The practice of using a medical device or drug in a way not specified in the original or current approved labeling is often referred to as "off-label" use.[92]
In regions where cardiac stenting has become commonplace, think tanks and advocacy groups express concern about the overzealous use of stents,[93] because patients who received stents for unapproved reasons[94][95] often have worse outcomes compared to patients who received stents for approved uses.[96][97][98]
Clinical procedure
DES placement
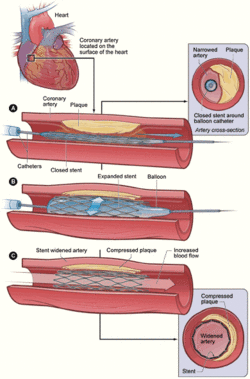
People who receive a coronary stent have different needs depending on their medical condition. Some patients are actually having a heart attack and need immediate life-saving emergency care. Other patients are at high risk of having a heart attack in the very near future. For people from each of these groups, PCI procedures may vary slightly, with particular modifications as to how they are sedated, pain management, and broader intensive care issues such as breathing support.[99]
Many people who are not in critical care situations are usually fully awake during the PCI procedure and DES placement, but they receive local anesthetic at the site of catheter entry, to ensure there is no pain. Different sedation and pain management practices are used by different medical institutions and practitioners, but patient comfort is always a primary consideration.[100]
The catheter/stent system is inserted into the body by piercing a peripheral artery (an artery in the arm or leg) and moved through the arterial system to deliver the DES into the blocked coronary artery. The stent is then expanded to widen (open) blocked or narrowed coronary arteries (narrowed by plaque buildup), caused by a condition called atherosclerosis. Peripheral arterial access is usually through the femoral (upper leg) or the radial artery (arm/wrist) and less often done through the brachial or ulnar artery (wrist/arm).[101][102] In the past, controlling bleeding at the point of arterial access after the procedure was a problem. Modern arterial pressure bands and arterial closure systems now exist, which have helped control bleeding after the procedure, but it is still a concern.[103][104][105]
Modern catheter/stent systems are integrated medical devices, made of a guidewire, catheter, balloon, and stent. The stent tube mesh is initially collapsed onto the balloon of the device, and it is small enough to be passed through relatively narrow peripheral arteries. When in position, the balloon is inflated by introducing physiological saline, and this pushes the overlaying stent firmly into the diseased artery wall, inflation time and pressure are recorded during this placement procedure. After placement, the balloon is deflated, and the device is removed from the body, leaving the expanded stent in place and opening up the artery.[67][106]
The interventional cardiologist decides how to treat the blockage in the best way during the PCI/DES placement, based on real-time data. The cardiologist uses imaging data provided by both intravascular ultrasound (IVUS), and fluoroscopic imaging (combined with a radiopaque dye). During the procedure, the information obtained from these two sources enables the cardiologist to track the path of the catheter-DES device as it moves through the arterial blood vessels. This information also helps determine both the location and characteristics of any plaque causing narrowing in the arteries. Data from these two techniques is used to correctly position the stent and to obtain detailed information relating to the coronary arterial anatomy. Given that this anatomy varies greatly among individuals, having this information becomes a prerequisite for effective treatment. The obtained data is recorded on video and may be used in cases when further treatment is needed.[107][108][109]
Post-stenting recovery and rehabilitation
For many people the stenting procedure does not require staying in the hospital for any extended time period, most people leave the hospital the same day. Much of the time immediately after the stenting is spent in a recovery area to make sure the access site is not bleeding and to ensure vital signs are stable.[110]
In most hospital settings, the interventional cardiologist who performed the procedure will speak directly with the patient/family and give them information about how things went, and follow-up instructions. The nursing staff will keep an eye on the person's condition and use tools like ECG to monitor their heart. To prevent a blood clot from forming in the stent, medications are given right after the procedure. One common medication is plavix, which is a potent blood thinner that comes as a pill. Other medicines that thin the blood are also used, and it's typical to combine aspirin with plavix.[111] For people who have had a heart attack, the length of hospitalization is dependent on the degree of heart muscle damage caused by the event.[112]
A catheter with DES is a medical device, so people who receive it are given a medical device card. This card has information on the implanted DES and a medical device serial number. This information is important for future medical procedures, because it helps the doctors to know what type of device is in the person's body. Some arterial closure systems, which are devices that help to seal the access site after the procedure, are also medical devices and have their own informational cards.[113]
The access site is the place where the catheter enters the artery in the arm or leg. There is usually soreness and bruising at this site. This bruising and soreness usually get better after a week or so. People are advised to rest for a week or two and not to lift heavy things. This is mainly to make sure the access site heals well. It is normal to have follow-up appointments with a cardiologist or a primary care provider/general practitioner within a week or two of the procedure.[114][115]
People who get a coronary stent usually have more check-ups every three to six months for the first year, but this can vary. They usually do not need to have another coronary angiography, which is a test that uses a special dye and X-rays to see the arteries of the heart. If the doctors suspect that the heart disease is getting worse, they can prescribe a stress test, which is a test that measures how the heart works during physical activity. People who have symptoms or show signs of reduced blood flow to the heart in a stress test may need to have a diagnostic cardiac re-catheterization.[116]
After PCI-stenting procedures, physical examinations are important. People who have a high risk of complications or more complex coronary problems may need to have angiography. This may be the case even if the results of non-invasive stress tests, which are tests that measure how the heart works during physical activity, appear normal.[117]
Cardiac rehabilitation activities depend on many factors, but mainly on how much the heart muscle was damaged before the PCI/DES procedure. Many people who have this procedure have not had a heart attack, and their hearts may be fine. Others may have had a heart attack and their hearts may have trouble pumping oxygen-rich blood to the body. Rehabilitation activities are tailored to each person's needs.[118]
Efficacy
Benefits
DES are an improvement over older BMS devices as they reduce the chances of in-stent blockages. This reduces the incidence of serious post-stenting events such as, angina occurrence or recurrence, heart attacks, and death. They also reduce the likelihood of requiring another PCI procedure to open a blockage caused by the actual stent.[70]
The major benefit of drug-eluting stents (DES) when compared to bare-metal stents (BMS) is the prevention of in-stent restenosis (ISR).[70] Restenosis is a gradual re-narrowing of the stented segment that occurs most commonly between 3–12 months after stent placement.[119] High rates of restenosis associated with BMS prompted the development of DES, which resulted in a reduction of ISR incidence to around 5-10%.[120] Continued development of newer generation DES have resulted in the near-elimination of BMS from clinical practice.[121]
Procedure outcomes
A key benefit of DES usage compared to BMS is a lower incidence of repeat revascularization procedures (re-stenting, invasive bypass surgeries etc.). Revascularization procedures are treatments that restore blood flow to parts of the heart that are not getting enough blood, a problem called ischemia. This can happen because of plaque buildup in the arteries of the heart, which can narrow or block them.[122] Drug-eluting stents reduce the risk of restenosis primarily by releasing anti-proliferative medications—such as sirolimus or paclitaxel—that inhibit neointimal hyperplasia, the excessive tissue growth inside the artery that can lead to re-narrowing. As a result, rates of repeat revascularizations and stent thrombosis (blood clots) are significantly lower in those who received DES compared to BMS.[120]
Newer generations of DES devices have substantially improved safety outcomes, specifically regarding stent thrombosis, recurrent myocardial infarctions, and death.[122] These improvements stem from advancements in stent design, including thinner struts, more biocompatible polymers, and enhanced drug-release kinetics. Such features promote better endothelial healing while maintaining anti-restenotic effects.
Considerations for regulatory submission, assessment and approval
There are a number of very detailed medical device design considerations for DES products, these considerations are included in submissions for approval to regulatory authorities such as the US FDA:[18]
- Aspects of the design that relate to a DES as structural devices that keep an artery open by purely physical means.
- Choice of the construction materials, with a particular focus on biocompatibility, longevity in the human body, mechanical stress resistance and the suitability of the chosen material for future patient imaging using MRI technologies, due to the high magnetic fields used in such imaging.[15]
- Choice of a mechanism of the drug release: how long the drug lasts, and how to make the stent release the drug in a manner that inhibits in-stent restenosis.
- Choice of chemical agent the stent will deliver.
- Choice of the stent delivery technology as an integrated system: catheter design, placement visualization and assessment of the success of artery reperfusion (is the treated artery actually supplying cardiac muscle with sufficient oxygenated blood).
- Quality assurance considerations such as those defined in ISO 13485.
- Quality control considerations: what testing can be performed on each manufactured unit prior to release for sale to demonstrate its usage suitability.[16][17][123]
- Traceability issues, can a single stent be traced from the manufacturer to the patient it was implanted in. In the case of a recall of a product it is critical to be able to trace the stent from design, manufacture, and distribution to the patient.
The drug choice is a critical design element and determining its true effectiveness in inhibiting neointimal growth due to the proliferation of smooth muscle cells that would cause restenosis can be a design challenge. Much of the neointimal hyperplasia seems to be caused by inflammation.[123]
Vascular stents are classified by the US as class III medical devices,[124] meaning that they pose the highest risk to patients and are subject to both general and premarket approval, which requires clinical trials and scientific evidence of safety and effectiveness, as well as rigorous mechanical testing.[125] During the mechanical testing process, universal testing machines induce bending, stretching, twisting, and putting pressure on vascular stents from various angles.[124]
The specific properties of each type of stent and its intended use depend on the results of testing, and vice versa: different types of stents may need different or additional tests based on where they will be placed in the body and what they will be used for. Some of these additional tests might include checking how well the stent can withstand being crushed or bent out of shape, its resistance to getting kinks in it, whether it resists corrosion or damage over time, as well as making sure any coatings on the device remain intact.[124]
Alternatives to stenting procedures
Pharmacological therapy for coronary artery disease may be indicated instead of or in addition to invasive treatment. For those requiring percutaneous coronary intervention or surgery, medical therapy should be viewed as complementary to revascularization procedures, rather than an opposing strategy. Coronary artery bypass graft (CABG) surgery is an alternative to percutaneous coronary intervention (PCI) with drug-eluting stents (DES) for patients with ischemic left ventricular systolic dysfunction (LVSD). CABG is associated with lower risks of all-cause mortality, repeat revascularization, and myocardial infarction compared to PCI.[126]
History
The first procedure to treat coronary occlusion was a coronary artery bypass graft (CABG), which uses a section of another vein or artery to bypass the obstructed segment of coronary artery. In 1977, Andreas Grüntzig introduced percutaneous transluminal coronary angioplasty (PTCA), also called balloon angioplasty, expanding a balloon catheter, guided through a peripheral artery, to force expansion of the narrowed segment.[127]
As equipment and techniques improved, the use of PTCA rapidly increased, and by the mid-1980s, PTCA and CABG were being performed at equivalent rates.[128] Balloon angioplasty was generally effective and safe, but restenosis was frequent, occurring in about 30–40% of cases, usually within the first year. In about 3% of balloon angioplasty cases, failure of the dilation and acute or threatened closure of the coronary artery (often because of dissection) prompted emergency CABGs.[128]
Charles Theodore Dotter and Melvin Judkins had proposed using prosthetic devices inside leg arteries to maintain blood flow after dilation as early as 1964.[129] In 1986, Puel and Sigwart implanted the first coronary stent in a human patient.[130] Several trials in the 1990s showed the superiority of stent placement over balloon angioplasty. Restenosis was reduced because the stent acted as a scaffold to hold open the dilated segment of the artery. Acute closure of the coronary artery (and the requirement for emergency CABG) was reduced, because the stent repaired dissections of the arterial wall. By 1999, stents were used in 84% of percutaneous coronary interventions (i.e., those done via a catheter, and not by open-chest surgery).[130]
Early difficulties with coronary stents included a risk of early thrombosis (clotting) resulting in occlusion of the stent.[128] Coating stainless steel stents with other substances such as platinum or gold did not eliminate this problem.[130] High-pressure balloon expansion of the stent to ensure its full apposition to the arterial wall,[jargon] combined with drug therapy using aspirin and another inhibitor of platelet aggregation (usually ticlopidine or clopidogrel) nearly eliminated this risk of early stent thrombosis.[128][130]
Though it occurred less frequently than with balloon angioplasty or other techniques, stents nonetheless remained vulnerable to restenosis, caused almost exclusively by neointimal tissue growth (tissue formation in the inner 'tube' structure of the artery). To address this issue, developers of drug-eluting stents used the devices themselves as a tool for delivering medication directly to the arterial wall. While initial efforts were unsuccessful, the release (elution) of drugs from the stent was shown in 2001 to achieve high concentrations of the drug locally, directly at the target lesion, with minimal systemic side effects.[131] As currently used in clinical practice, "drug-eluting" stents refers to metal stents that elute a drug designed to limit the growth of neointimal scar tissue, thus reducing the likelihood of stent restenosis.[132]
The first type of DES to be approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) were sirolimus-eluting stents (SES), releasing sirolimus,[133] a natural immunosuppressant drug.[134] SES were shown to reduce the need for repeat procedures and improve the outcomes of patients with coronary artery disease.[135][136][137] The sirolimus-eluting Cypher stent received CE mark approval in Europe in 2002, and then underwent a larger trial to demonstrate its safety and effectiveness for the US market.[138][139][140] The trial, published in 2003, enrolled 1058 patients with more complex lesions and confirmed the superiority of SES over bare metal stents in terms of angiographic and clinical outcomes.[141][142][143][144] Based on these results, the Cypher stent received FDA approval and was released in the US in 2003.[130] The FDA approval process for DES involves submitting an investigational device exemption (IDE) application to conduct clinical trials under 21 CFR Part 812, and then a premarket approval (PMA) application to obtain marketing authorization under 21 CFR Part 8144. The FDA assigns the primary review responsibility to the Center for Devices and Radiological Health (CDRH), but also consults with the Center for Drug Evaluation and Research (CDER) for the drug component of the combination product.
The second type of DES to be approved by the EMA and the FDA were paclitaxel-eluting stents (PES), which release another natural product called paclitaxel. PES also reduced the need for repeat procedures and improved the outcomes of patients with different types of lesions and risk factors. The paclitaxel-eluting Taxus stent received FDA approval and was launched in the US in 2004,[145] after a series of trials that compared it with a bare metal stent in various settings. The trials showed a significant reduction in target lesion revascularization and major adverse cardiac events with the Taxus stent at 9 and 12 months. Both SES and PES use natural products as the active agents to prevent the recurrence of blockages in the arteries.[146] These DES have changed the practice of interventional cardiology and have become the preferred treatment for many patients with coronary artery disease.[146][147][148]
The initial rapid acceptance of DES led to their peak usage in 2005, accounting for 90% of all stent implantations, but concerns about late stent thrombosis led to a decrease in DES usage in late 2006. Subsequent studies reassured the medical community about their safety, showing that while DES may have a slightly higher risk for very late stent thrombosis, they significantly reduce target vessel revascularization without increasing the incidence of death or myocardial infarction; these reassurances led to a resurgence in DES utilization, although it did not reach the peak usage rates seen in early 2006.[149][150]
The concept of using absorbable (also called biodegradable, bioabsorbable or bioresorbable)[151] materials in stents was first reported in 1878 by Huse who used magnesium wires as ligatures to halt the bleeding in vessels of three patients. Despite extensive search, the full name of this pioneer in the field remains elusive.[151][152] In 20th century, a resorbable stent tested in humans was developed by the Igaki Medical Planning Company in Japan and was constructed from poly-L-lactic acid (a form of polylactic acid); they published their initial results in 2000.[153] The German company Biotronik developed a magnesium absorbable (bioresorbable) stent and published clinical results in 2007.[153]
The first company to bring a bioresorbable stent to market was Abbott Vascular which received European marketing approval in September 2012; the second was Elixir which received its CE mark in May 2013.[154][155][156]
Despite the initial promise, the first-generation bioresorbable stents, such as the Absorb bioresorbable stent by Abbott, faced significant challenges in their performance. In comparison to current-generation drug-eluting stents, numerous trials revealed that these first-generation bioresorbsble stents exhibited poor outcomes. Specifically, they showed high rates of stent thrombosis (cases where an implanted coronary stent caused a thrombotic occlusion), target-lesion myocardial infarction (heart attack occurring at the site of the treated lesion), and target vessel revascularization (the need for further procedures to restore blood flow in the treated artery). In 2017, Abbott pulled its bioabsorbable stent, Absorb, from the European market after negative press regarding the device.[157] Boston Scientific also announced termination of its Renuvia bioresorbable coronary stent program as studies showed higher risk of serious adverse events.[158]
Currently, fully bioresorbable stents do not play a significant role in coronary interventions.[159][160][161] While various manufacturers are proposing new stents and continuing their development,[162] it remains uncertain whether they will have a substantial impact, unless there will be more data from their clinical trials. As of now, these stents are not widely utilized in practice.[163][164][159]
Due to challenges in developing resorbable stents, many manufacturers have focused efforts on targeting or reducing drug release through bioabsorbable-polymer coatings. Boston Scientific's Synergy bioabsorbable polymer stent has been shown potential to reduce the length of dual antiplatelet therapy post-implantation.[165] MicroPort's Firehawk target eluting stent has been shown to be non-inferior to traditional drug-eluting stents while using one-third of the amount of equivalent drug.[166]
As for the materials used to make a DES, the first DES products available for treating patients were stainless steel alloys composed of iron, nickel, and chromium and were based on existing bare metal stents.[123] These stents were hard to visualize with medical imaging, posed a risk of causing allergic responses, and were difficult to deliver. Subsequent new alloys were used, namely cobalt-chrome and platinum chrome, with improved performance. Bioresorbable stents have been developed in which the stent itself dissolves over time.[58] Materials explored for use include magnesium, polylactic acid, polycarbonate polymers, and salicylic acid polymers.[153] Resorbable stents have held the promise of providing an acute treatment that would eventually allow the vessel to function normally, without leaving a permanent device behind.[167]
For the coating of DES, one to three or more layers of polymer can be used: a base layer for adhesion, a main layer that holds and elutes (releases) the drug into the arterial wall by contact transfer, and sometimes a top coat to slow down the release of the drug and extend its effect. The first few drug-eluting stents to be licensed used durable coatings. The first generation of coatings appears to have caused immunological reactions at times, and some possibly led to thrombosis. This has driven experimentation and the development of new coating approaches.[154]
Research directions
A research direction for a DES is to improve the material from which a device is made. The first-generation DES were made of stainless steel, while contemporary DES mainly consist of different kinds of alloys such as cobalt chromium and platinum chromium. In the current generation DES, thinner struts are employed than in the first-generation DES with preserved radial strength and radio-opacity. The lower strut thickness is believed to be associated with better stent-related outcomes including target lesion revascularization, myocardial infarction, and stent thrombosis.[168]
Another area of research for DES focuses on polymers. The current generation DES includes both durable polymer-coated stents and biodegradable polymer-coated stents. It has been reported that the presence of a durable polymer in the body over a long period can lead to chronic inflammation and neoatherosclerosis. To address this potential limitation, researchers have developed biodegradable polymer DES as an alternative solution.[168][169][170]
Scientists are also studying different drugs that could be used in DES to prevent restenosis. These drugs, which have immunosuppressive[134] and anti-cancer properties, aim to inhibit the growth of smooth muscle cells. Additionally, there is a specific type of stent that features an extra layer of anti-CD4 antibodies on its struts. This additional layer is positioned on top of the polymer coating and aims to capture circulating endothelial progenitor cells. The goal behind this design is to promote improved healing of the blood vessel lining, known as the endothelium.[168][61]
A potential research focus for DES is the application of a polymer-free DES in clinical practice: moving away from polymer-based DES and instead using either a polymer-free DES or a drug-coated coronary stent. In the case of the polymer-free DES, it utilizes an abluminal coating of probucol to control the release of sirolimus. On the other hand, the drug-coated coronary stent has a micro-structured abluminal surface that allows for direct application of an anti-restenotic drug.[168][61]
Society and culture
Brand names and manufacturers
As of 2023[update] there are over 20 different types of drug-eluting stents available, with differences in features and characteristics.[171]
Economics
The economic evaluation of DES has been a topic of extensive research.[172] In 2007, the overall incremental cost-effectiveness ratio in Europe was €98,827 per quality-adjusted life-years gained. Avoiding one revascularization with DES would cost €4,794, when revascularization with BMS costs €3,2606.[173]
Controversies
There were controversies related to the use of DES. In 2012, a meta-analysis of clinical trial data[174] showed no benefit of the use of DES for people with stable coronary artery compared to treatment with drugs, yet, The New York Times interviewed David Brown, an author of the analysis, who said that more than half of patients with stable coronary artery disease were implanted with stents without even trying drug treatment and that he believed this happened because hospitals and doctors wanted to make more money.[175]
The interview sparked a debate among cardiologists, researchers, and patients about the appropriateness and effectiveness of DES for stable coronary artery disease: some agreed with the study's findings and questioned the overuse of stents,[176][177][178] while others criticized the study's methods and limitations and defended the benefits of stents, arguing that the interviewee's statement was "outrageous and defamatory" and that he was "insulting the integrity of the entire profession.[179][180][181]
In 2013 the Times of India reported that DES were widely overused and that Indian distributors used profits from high markups on DES to bribe doctors to use them.[182][183]
In 2014 an investigation by the Maharashtra Food and Drug Administration found that high markups and bribery related to DES was still widespread.[184]
More recent research and clinical guidelines have clarified the role of DES in stable coronary artery disease. While large trials and meta-analyses have confirmed that DES do not reduce mortality or myocardial infarction risk in stable patients compared to optimal medical therapy, they can provide significant relief for patients who continue to experience angina despite pharmacologic treatment. As a result, current guidelines emphasize individualized assessment, shared decision-making, and reserving PCI with DES for patients whose symptoms are not adequately controlled with medications.[185][186]
Intellectual property disputes
There have been several patent disputes related to drug-eluting stents. In one of them, Boston Scientific Corporation (BSC) has been found guilty of infringing upon a patent awarded to the University of Texas at Arlington in 2003 and licensed to TissueGen.[187][188][189] This patent involves technology developed by TissueGen founder Kevin Nelson, during his time as a faculty member at the university. The technology is designed to deliver drugs through an extruded fiber within an implanted vascular stent. As a result, BSC has been ordered to pay $42 million in lost royalties to both TissueGen and the university[187][188]
Class action lawsuits
Drug-eluting stents have been associated with legal and ethical controversies, and there have been related class action lawsuits. In 2014, the former owners of St. Joseph Medical Center in Maryland settled a class action lawsuit for $37 million with hundreds of patients who received unnecessary DES implantation. The lawsuit alleged that Dr. Mark Midei, a cardiologist at the center, falsified the degree of coronary artery stenosis to justify the use of DES, exposing the patients to increased risks of thrombosis, bleeding, and infection. Another DES manufacturer, Cordis Corporation, a subsidiary of Johnson & Johnson, was involved in lawsuits from people who suffered adverse events from the Cypher Stent, a stainless-steel DES coated with sirolimus,[190][191] an immunosuppressant drug.[134] The Cypher Stent was approved by the FDA in 2003, but soon after, the FDA issued a Safety Warning following 290 reports of subacute thrombosis and at least 60 deaths related to the device.[190][191]
See also
References
- ↑ "Stent: MedlinePlus Medical Encyclopedia". https://medlineplus.gov/ency/article/002303.htm.
- ↑ "Drug-Eluting Stents Information". https://www.columbiadoctors.org/health-library/article/drug-eluting-stents/.
- ↑ "Drug Eluting Stent Compounds". StatPearls. 2024. https://www.ncbi.nlm.nih.gov/books/NBK537349/.
- ↑ "Drug-eluting Stents - Current and Future Perspectives" (in en). Interventional Cardiology 1 (1): 28–29. 2006. doi:10.15420/icr.2006.1.1.28. https://www.icrjournal.com/articles/drug-eluting-stents-current-and-future-perspectives. Retrieved 20 November 2023.
- ↑ "Onyx Frontier DES - Coronary Stents" (in en). Medtronic. https://www.medtronic.com/us-en/healthcare-professionals/products/cardiovascular/stents/onyx-frontier-des.html.
- ↑ "Promus PREMIER™ Drug-Eluting Coronary Stent System" (in en-us). https://www.bostonscientific.com/en-US/products/stents--coronary/promus-premier-stent-system.html.
- ↑ 7.0 7.1 7.2 7.3 7.4 Velagapudi, C; Madassery, S (November 2022). "Drug-eluting stents". Semin Intervent Radiol 39 (4): 400–405. doi:10.1055/s-0042-1758078. PMID 36406018.
- ↑ "Coronary stents: historical development, current status and future directions". British Medical Bulletin 106: 193–211. 2013. doi:10.1093/bmb/ldt009. PMID 23532779.
- ↑ 9.0 9.1 "Clinical outcomes for single stent and multiple stents in contemporary practice". Clin Cardiol 32 (9): E33–9. September 2009. doi:10.1002/clc.20516. PMID 19645042.
- ↑ 10.0 10.1 "Multiple overlapping drug-eluting stents to treat diffuse disease of the left anterior descending coronary artery". J Am Coll Cardiol 45 (10): 1570–3. May 2005. doi:10.1016/j.jacc.2005.01.049. PMID 15893168.
- ↑ "Efficacy of intravascular imaging-guided drug-eluting stent implantation: A systematic review and meta-analysis of randomized clinical trials". BMC Cardiovascular Disorders 22 (1). 2022. doi:10.1186/s12872-022-02772-w. PMID 35870904.
- ↑ "Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients". BMC Cardiovascular Disorders 18 (1). 2018. doi:10.1186/s12872-018-0843-z. PMID 29898668.
- ↑ "Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries". PLOS ONE 18 (9). 2023. doi:10.1371/journal.pone.0291466. PMID 37733656. Bibcode: 2023PLoSO..1891466L.
- ↑ "Different drugs in drug-eluting stents for peripheral artery disease: A systematic evaluation and Bayesian meta-analysis". Journal of Thrombosis and Thrombolysis 57 (3): 520–530. 2024. doi:10.1007/s11239-023-02932-5. PMID 38281227. https://link.springer.com/article/10.1007/s11239-023-02932-5. Retrieved 11 March 2024.
- ↑ 15.0 15.1 15.2 15.3 "Materials for metallic stents". Journal of Artificial Organs 12 (2): 73–79. 1 June 2009. doi:10.1007/s10047-008-0456-x. PMID 19536623.
- ↑ 16.0 16.1 16.2 16.3 "Absorbable stent: focus on clinical applications and benefits". Vascular Health and Risk Management 8: 125–132. 2012. doi:10.2147/VHRM.S22551. PMID 22399857.
- ↑ 17.0 17.1 17.2 17.3 ""The Unpredictable ABSORB" - Very Late Stent Thrombosis of Bioresorbable Vascular Scaffold". Heart Views 20 (2): 65–69. 2019. doi:10.4103/HEARTVIEWS.HEARTVIEWS_18_19. PMID 31462962.
- ↑ 18.0 18.1 18.2 18.3 "The Newest Generation of Drug-eluting Stents and Beyond". European Cardiology 13 (1): 54–59. August 2018. doi:10.15420/ecr.2018:8:2. PMID 30310472. PMC 6159420. https://www.ecrjournal.com/articles/newest-generation-drug-eluting-stents-and-beyond. Retrieved 21 October 2023.
- ↑ "Optimization of Drug Delivery by Drug-Eluting Stents". PLOS ONE 10 (6). 2015. doi:10.1371/journal.pone.0130182. PMID 26083626. Bibcode: 2015PLoSO..1030182B.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 "Critical evaluation of stents in coronary angioplasty: A systematic review". BioMedical Engineering OnLine 20 (1). 2021. doi:10.1186/s12938-021-00883-7. PMID 33964954.
- ↑ "Efficacy and Safety of Biodegradable Polymer Biolimus-Eluting Stents versus Durable Polymer Drug-Eluting Stents: A Meta-Analysis". PLOS ONE 8 (11). 2013. doi:10.1371/journal.pone.0078667. PMID 24244335. Bibcode: 2013PLoSO...878667Y.
- ↑ "Comparison of biodegradable and durable polymer drug-eluting stents in acute coronary syndrome: A meta-analysis". BMJ Open 12 (6). 2022. doi:10.1136/bmjopen-2021-058075. PMID 35676012. PMC 9185674. https://bmjopen.bmj.com/content/12/6/e058075. Retrieved 11 March 2024.
- ↑ "Efficacy and safety of polymer-free stent versus polymer-permanent drug-eluting stent in patients with acute coronary syndrome: A meta-analysis of randomized control trials". BMC Cardiovascular Disorders 17 (1). 2017. doi:10.1186/s12872-017-0603-5. PMID 28724348.
- ↑ "Contemporary coronary drug-eluting and coated stents: An updated mini-review (2023)". Cardiovascular Intervention and Therapeutics 39 (1): 15–17. 2024. doi:10.1007/s12928-023-00954-7. PMID 37656338. https://link.springer.com/article/10.1007/s12928-023-00954-7. Retrieved 11 March 2024.
- ↑ Watson T; Webster MWI (2017). "Long and short of optimal stent design". Open Heart 4 (2). doi:10.1136/openhrt-2017-000680. PMID 29118997.
- ↑ "Stent Design: Implications for Restenosis". Reviews in Cardiovascular Medicine 3 (S5): 16–22. 20 November 2002. https://www.imrpress.com/journal/RCM/3/S5/pii/1561516747581-954903891. Retrieved 11 March 2024.
- ↑ "Comparison of drug-eluting balloon versus drug-eluting stent for treatment of coronary artery disease: A meta-analysis of randomized controlled trials". BMC Cardiovascular Disorders 18 (1). 2018. doi:10.1186/s12872-018-0771-y. PMID 29499651.
- ↑ "Drug-coated balloons versus drug-eluting stents in patients with acute myocardial infarction undergoing percutaneous coronary intervention: An updated meta-analysis with trial sequential analysis". BMC Cardiovascular Disorders 23 (1). 2023. doi:10.1186/s12872-023-03633-w. PMID 38066453.
- ↑ Coronary Artery Disease and the Evolution of Angioplasty Devices. SpringerBriefs in Materials. 2020. doi:10.1007/978-3-030-42443-5. ISBN 978-3-030-42442-8.
- ↑ "Overview of cardiovascular stent designs". Functionalised Cardiovascular Stents. 2018. pp. 3–26. doi:10.1016/B978-0-08-100496-8.00001-9. ISBN 978-0-08-100496-8. http://doi.org/10.1016/B978-0-08-100496-8.00001-9. Retrieved 11 March 2024.
- ↑ "50 Drug-eluting balloons and drug-eluting stents in the treatment of small coronary arteries: A systematic review and meta-analysis of long-term clinical outcomes". General posters. 2022. pp. A43–A46. doi:10.1136/heartjnl-2022-ICS.50. https://doi.org/10.1136/heartjnl-2022-ICS.50. Retrieved 11 March 2024.
- ↑ "Coronary Angioplasty and Drug-Eluting Stents". Applications of Biotechnology in Cardiovascular Therapeutics. 2011. pp. 259–313. doi:10.1007/978-1-61779-240-3_9. ISBN 978-1-61779-239-7. https://doi.org/10.1007/978-1-61779-240-3_9. Retrieved 11 March 2024.
- ↑ "Percutaneous Coronary Intervention (PCI)" (in en). https://www.yalemedicine.org/conditions/percutaneous-coronary-intervention-pci.
- ↑ Medtronic. "Onyx Frontier DES - Coronary Stents" (in en). https://www.medtronic.com/us-en/healthcare-professionals/products/cardiovascular/stents/onyx-frontier-des.html.
- ↑ "Antiproliferative Drug-Eluting Stents: Systematic Review of the Benefits and Estimate of Economic Impact". Revista Española de Cardiología (English Edition) 57 (7): 617–628. 2004. doi:10.1016/S1885-5857(06)60285-5. PMID 15274846.
- ↑ "Drug-eluting stents: An early systematic review to inform policy*1, *2". European Heart Journal 25 (11): 902–919. 2004. doi:10.1016/j.ehj.2004.03.023. PMID 15172462.
- ↑ "Local Delivery of Antiproliferative Agents via Stents". Polymers 6 (3): 755–775. 2014. doi:10.3390/polym6030755.
- ↑ "Drug-Eluting Stents" (in en). https://www.uspharmacist.com/article/drug-eluting-stents.
- ↑ "Assessment and management of peripheral arterial disease: What every cardiologist should know". Heart 107 (22): 1835–1843. 2021. doi:10.1136/heartjnl-2019-316164. PMID 33985986.
- ↑ "Structural and Hemodynamic Analyses of Different Stent Structures in Curved and Stenotic Coronary Artery". Frontiers in Bioengineering and Biotechnology 7. 2019. doi:10.3389/fbioe.2019.00366. PMID 31867313.
- ↑ "Coronary Artery Stents". JAMA 284 (14): 1828–1836. 2000. doi:10.1001/jama.284.14.1828. PMID 11025836. https://jamanetwork.com/journals/jama/fullarticle/193148. Retrieved 11 March 2024.
- ↑ Mao, H; Bao, J (2018). "Peripheral Stent". in Jing, Z; Mao, H. Endovascular Surgery and Devices. Springer. pp. 43–57. doi:10.1007/978-981-10-8270-2_5. ISBN 978-981-10-8270-2. https://link.springer.com/book/10.1007/978-981-10-8270-2. Retrieved 5 March 2024.
- ↑ "Comparison of biodegradable and durable polymer drug-eluting stents in acute coronary syndrome: A meta-analysis". BMJ Open 12 (6). 2022. doi:10.1136/bmjopen-2021-058075. PMID 35676012.
- ↑ 44.0 44.1 "Atherosclerosis | Description, Pathophysiology, Risk Factors, & Treatment | Britannica". 13 March 2024. https://www.britannica.com/science/atherosclerosis.
- ↑ 45.0 45.1 45.2 "Drug-eluting stents for coronary artery disease in the perspective of bibliometric analysis". Frontiers in Cardiovascular Medicine 11. 2024. doi:10.3389/fcvm.2024.1288659. PMID 38440210.
- ↑ "New Generations of Drug-Eluting Stents – A Brief Review". EMJ Interventional Cardiology: 100–106. 2014. doi:10.33590/emjintcardiol/10312250.
- ↑ "Drug-eluting stents for the treatment of coronary artery disease: A review of recent advances". Expert Opin Drug Deliv 19 (3): 269–280. March 2022. doi:10.1080/17425247.2022.2044784. PMID 35180832. https://ir.ymlib.yonsei.ac.kr/handle/22282913/188391.
- ↑ "Coronary-Artery Stents". New England Journal of Medicine 354 (5): 483–495. 2 February 2006. doi:10.1056/NEJMra051091. PMID 16452560. https://www.nejm.org/doi/pdf/10.1056/NEJMra051091. Retrieved 15 March 2024.
- ↑ Katib, N; Varcoe, RL (2023). "Intervention for chronic lower limb ischemia". in Loftus, I; Hinchliffe, RJ. Vascular and Endovascular Surgery (7th ed.). Elsevier Health Sciences. pp. 39–57. ISBN 978-0-7020-8463-8. https://books.google.com/books?id=l_7KEAAAQBAJ. Retrieved 4 March 2024.
- ↑ Nordanstig, J et al. (January 2024). "Editor's Choice -- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication". Eur J Vasc Endovasc Surg 67 (1): 9–96. doi:10.1016/j.ejvs.2023.08.067. PMID 37949800. https://www.ejves.com/action/showPdf?pii=S1078-5884%2823%2900741-4. Retrieved 3 March 2024.
- ↑ 51.0 51.1 51.2 "Different drugs in drug-eluting stents for peripheral artery disease: A systematic evaluation and Bayesian meta-analysis". Journal of Thrombosis and Thrombolysis 57 (3): 520–530. 2024. doi:10.1007/s11239-023-02932-5. PMID 38281227.
- ↑ 52.0 52.1 52.2 52.3 "Drug-Eluting Stents for Treatment of Peripheral Artery Disease". American Journal of Cardiovascular Drugs 18 (3): 175–180. 2018. doi:10.1007/s40256-018-0265-4. PMID 29737505.
- ↑ 53.0 53.1 53.2 53.3 "Drug-eluting stents in the management of peripheral arterial disease". Vascular Health and Risk Management 4 (3): 553–559. 2008. doi:10.2147/VHRM.S1712. PMID 18827906.
- ↑ "Fabrication and application of drug eluting stent for peripheral artery disease". Korean Journal of Chemical Engineering 40 (2): 361–368. 2023. doi:10.1007/s11814-022-1286-x.
- ↑ 55.0 55.1 55.2 55.3 "Structural Design of Vascular Stents: A Review". Micromachines 12 (7): 770. 2021. doi:10.3390/mi12070770. PMID 34210099.
- ↑ "Overview of the latest developments in the field of drug-eluting stent technology". Biomater Sci 8 (2): 544–551. January 2020. doi:10.1039/c9bm00468h. PMID 31701961.
- ↑ "Efficacy of Intravascular Imaging-Guided Drug-Eluting Stent Implantation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials". Curr Probl Cardiol 49 (1 Pt A). January 2024. doi:10.1016/j.cpcardiol.2023.102002. PMID 37544623.
- ↑ 58.0 58.1 "A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds". Cleveland Clinic Journal of Medicine 84 (12 Suppl 4): e20–e24. December 2017. doi:10.3949/ccjm.84.s4.05. PMID 29281608.
- ↑ 59.0 59.1 "Vascular stents. Expanding use". Scientific American 295 (1): 94–95. July 2006. doi:10.1038/scientificamerican0706-94. PMID 16830686.
- ↑ "Dual Anti-platelet Therapy after Coronary Stenting: Rationale for Personalized Duration of Therapy" (in en). US Cardiol Rev 11: 31–36. 15 February 2017. doi:10.15420/usc.2017:7:2. https://www.uscjournal.com/articles/dual-anti-platelet-therapy-after-coronary-stenting-rationale-personalized-duration-therapy. Retrieved 20 November 2023.
- ↑ 61.0 61.1 61.2 "Drug-Eluting Stents: Technical and Clinical Progress". Biomimetics 8 (1): 72. February 2023. doi:10.3390/biomimetics8010072. PMID 36810403.
- ↑ "Drug-eluting stents: preventing restenosis". Cardiology in Review 15 (1): 1–12. 2007. doi:10.1097/01.crd.0000200844.16899.fc. PMID 17172878.
- ↑ "Drug-Eluting Coronary Artery Stents". American Family Physician 80 (11): 1245–1251. December 2009. PMID 19961137. https://www.aafp.org/pubs/afp/issues/2009/1201/p1245.html. Retrieved 15 March 2024.
- ↑ "UpToDate". https://www.uptodate.com/contents/intracoronary-stents-stent-types.
- ↑ "Percutaneous Coronary Intervention and Bleeding Complications" (in en-GB). EMJ Interventional Cardiology 4 (1): 100–109. 30 June 2016. doi:10.33590/emjintcardiol/10314557. ISSN 2053-423X. https://www.emjreviews.com/interventional-cardiology/article/percutaneous-coronary-intervention-and-bleeding-complications/. Retrieved 19 November 2023.
- ↑ "Percutaneous Coronary Intervention". StatPearls. Treasure Island (FL): StatPearls Publishing. 2023. https://www.ncbi.nlm.nih.gov/books/NBK556123/. Retrieved 21 November 2023.
- ↑ 67.0 67.1 "Percutaneous Coronary Intervention (PCI)" (in en). https://www.yalemedicine.org/conditions/percutaneous-coronary-intervention-pci.
- ↑ "Contemporary Drug-Eluting Stents and Vascular Response" (in en-GB). EMJ Interventional Cardiology 2 (1): 60–68. 3 February 2017. doi:10.33590/emj/10314324. ISSN 2053-423X. https://www.emjreviews.com/interventional-cardiology/article/contemporary-drug-eluting-stents-and-vascular-response/. Retrieved 5 November 2023.
- ↑ "Evolving Coronary Stent Technologies - A Glimpse Into the Future". Cureus 15 (3). March 2023. doi:10.7759/cureus.35651. PMID 37009355.
- ↑ 70.0 70.1 70.2 70.3 "Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials" (in English). Lancet 393 (10190): 2503–2510. June 2019. doi:10.1016/S0140-6736(19)30474-X. PMID 31056295.
- ↑ "Advantages of DES over BMS in Preventing the Risk of Myocardial Infarction, Ischemic Stroke, and Mortality in Various Populations". Journal of Clinical Medicine 12 (1): 24. December 2022. doi:10.3390/jcm12010024. PMID 36614825.
- ↑ "2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation 145 (3): e18–e114. January 2022. doi:10.1161/CIR.0000000000001038. PMID 34882435.
- ↑ "Current Indications for Stenting: Symptoms or Survival CME". Methodist DeBakey Cardiovascular Journal 14 (1): 7–13. 2018. doi:10.14797/mdcj-14-1-7. PMID 29623167.
- ↑ "Atypical chest pain: a typical humpty dumpty coinage". Texas Heart Institute Journal 36 (5): 373–374. 2009. PMID 19876411.
- ↑ "Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes". JAMA 294 (20): 2623–2629. November 2005. doi:10.1001/jama.294.20.2623. PMID 16304077.
- ↑ "Coronary Artery Disease" (in en-us). U.S. Centers for Disease Control and Prevention (CDC). 19 July 2021. https://www.cdc.gov/heartdisease/coronary_ad.htm.
- ↑ "Patient education: Stenting for the heart (Beyond the Basics)". UpToDate. 19 June 2023. https://www.uptodate.com/contents/stenting-for-the-heart-beyond-the-basics/print.
- ↑ "Angioplasty and Stent Insertion" (in en-AU). 18 August 2016. https://www.insideradiology.com.au/angioplasty-and-stent-insertion-hp/.
- ↑ "Better stent expansion by two-time inflation of stent balloon and its responsible mechanism". Journal of Cardiology 59 (2): 160–166. March 2012. doi:10.1016/j.jjcc.2011.12.003. PMID 22266460.
- ↑ (in English) Manual of Cardiovascular Medicine (5th ed.). Philadelphia: Lippincott Williams & Wilkins. 2013. pp. 929–949. ISBN 978-1-4963-1260-0.
- ↑ "Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis? -a review of the evidences on coronary artery disease". Annals of Cardiothoracic Surgery 7 (4): 506–515. July 2018. doi:10.21037/acs.2018.05.17. PMID 30094215.
- ↑ "Percutaneous coronary intervention without on-site surgical back-up; two-years registry of a large Dutch community hospital". International Journal of Cardiology 132 (1): 59–65. February 2009. doi:10.1016/j.ijcard.2007.10.037. PMID 18241941.
- ↑ "New Statement Shows PCI Without Surgery on Site Is as Safe as PCI With Surgery on Site". Society for Cardiovascular Angiography & Interventions (SCAI). https://scai.org/new-statement-shows-pci-without-surgery-site-safe-pci-surgery-site.
- ↑ "How to minimize stent thrombosis". Circulation 124 (11): 1283–1287. September 2011. doi:10.1161/CIRCULATIONAHA.110.976829. PMID 21911796.
- ↑ "Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach". Journal of Cardiothoracic Surgery 17 (1). April 2022. doi:10.1186/s13019-022-01812-y. PMID 35379273.
- ↑ "Percutaneous Coronary Intervention". Cardiology Secrets. Elsevier. 2018. pp. 172–182. doi:10.1016/B978-0-323-47870-0.00019-2. ISBN 978-0-323-47870-0. "8 What is stent thrombosis? Stent thrombosis occurs when there is complete occlusion of the artery due to the formation of a thrombus in the stent. ... Stent thrombosis is a potentially catastrophic event and often presents as STEMI, requiring emergency revascularization. Stent thrombosis carries a mortality rate of 20% to 45%."
- ↑ "Long-term clinical outcomes following coronary stenting". Archives of Internal Medicine 168 (15): 1647–1655. August 2008. doi:10.1001/archinte.168.15.1647. PMID 18695078.
- ↑ "Poly(diol-co-citrate)s as novel elastomeric perivascular wraps for the reduction of neointimal hyperplasia". Macromolecular Bioscience 11 (5): 700–709. May 2011. doi:10.1002/mabi.201000509. PMID 21341372.
- ↑ "Intravascular Imaging-Guided Percutaneous Coronary Intervention: A Universal Approach for Optimization of Stent Implantation". Circulation: Cardiovascular Interventions 13 (12). December 2020. doi:10.1161/CIRCINTERVENTIONS.120.008686. PMID 33233934.
- ↑ "Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China". European Journal of Medical Research 27 (1). January 2022. doi:10.1186/s40001-022-00640-z. PMID 35065663.
- ↑ "Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts". Catheterization and Cardiovascular Interventions 66 (4): 507–511. December 2005. doi:10.1002/ccd.20498. PMID 16270361.
- ↑ "Comparison of drug-eluting and bare metal stents for saphenous vein graft lesions (from the National Heart, Lung, and Blood Institute Dynamic Registry)". The American Journal of Cardiology 106 (7): 946–951. October 2010. doi:10.1016/j.amjcard.2010.05.025. PMID 20854955.
- ↑ "PRESS RELEASE: Unnecessary coronary stents cost Medicare as much as $800 million per year" (in en-US). 31 October 2023. https://lowninstitute.org/press-release-unnecessary-coronary-stents-cost-medicare-as-much-as-800-million-per-year/.
- ↑ "Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents". JAMA 297 (18): 2001–2009. May 2007. doi:10.1001/jama.297.18.2001. PMID 17488965.
- ↑ "Outcomes and complications associated with off-label and untested use of drug-eluting stents". JAMA 297 (18): 1992–2000. May 2007. doi:10.1001/jama.297.18.1992. PMID 17488964.
- ↑ "Avoiding Overuse: Coronary Stents" (in en-US). https://lownhospitalsindex.org/avoiding-coronary-stent-overuse/.
- ↑ "The year in interventional cardiology". Journal of the American College of Cardiology 55 (20): 2272–86. May 2010. doi:10.1016/j.jacc.2010.02.024. PMID 20466207.
- ↑ "On-label and off-label use of drug-eluting stents: comparison of short- and long-term outcomes". Texas Heart Institute Journal 39 (1): 24–29. 2012. PMID 22412223.
- ↑ "Percutaneous coronary intervention" (in en). https://www.heartandstroke.ca/en/heart-disease/treatments/surgery-and-other-procedures/percutaneous-coronary-intervention/.
- ↑ "Monitored Anesthesia Care for Cardiovascular Interventions". Korean Circulation Journal 50 (1): 1–11. January 2020. doi:10.4070/kcj.2019.0269. PMID 31642214.
- ↑ "Percutaneous Coronary Intervention (PCI) Technique: Access, Procedure, Anatomic and Physiologic Assessment" (in en). 27 November 2019. https://emedicine.medscape.com/article/161446-technique?form=fpf.
- ↑ "TR BAND® Radial Compression Device" (in en). https://www.terumois.com/products/closure/tr-band.html.
- ↑ "Bleeding after percutaneous coronary intervention: can we still ignore the obvious?". Open Heart 1 (1). 28 February 2014. doi:10.1136/openhrt-2014-000036. PMID 25332793.
- ↑ "Access-Site vs Non-Access-Site Major Bleeding and In-Hospital Outcomes Among STEMI Patients Receiving Primary PCI". CJC Open 3 (7): 864–871. July 2021. doi:10.1016/j.cjco.2021.02.009. PMID 34401693.
- ↑ "40 Years of Percutaneous Coronary Intervention: History and Future Directions". Journal of Personalized Medicine 8 (4): 33. October 2018. doi:10.3390/jpm8040033. PMID 30275411.
- ↑ "Onyx Frontier DES - Coronary Stents" (in en). Medtronic. https://www.medtronic.com/us-en/healthcare-professionals/products/cardiovascular/stents/onyx-frontier-des.html.
- ↑ "IVUS in PCI Guidance". https://www.acc.org/latest-in%20cardiology/articles/2016/06/13/10/01/ivus-in-pci-guidance.
- ↑ Center for Devices and RadiologicalHealth (15 August 2023). "Fluoroscopy" (in en). FDA. https://www.fda.gov/radiation-emitting-products/medical-x-ray-imaging/fluoroscopy. Retrieved 21 November 2023.
- ↑ "Iodine-containing contrast medium" (in en-AU). 13 September 2016. https://www.insideradiology.com.au/iodine-containing-contrast-medium-hp/.
- ↑ "Management of patients after primary percutaneous coronary intervention for myocardial infarction". BMJ 358. July 2017. doi:10.1136/bmj.j3237. PMID 28729460.
- ↑ "Angioplasty and Vascular Stenting" (in en). https://www.radiologyinfo.org/en/info/angioplasty.
- ↑ "Short Hospital Stays After Angioplasty Following Heart Attack Often Sufficient". https://www.acc.org/About-ACC/Press-Releases/2015/03/23/14/51/http%3a%2f%2fwww.acc.org%2fAbout-ACC%2fPress-Releases%2f2015%2f03%2f23%2f14%2f51%2fShort-Hospital-Stays-After-Angioplasty-Following-Heart-Attack-Often-Sufficient.
- ↑ "Coronary angioplasty and stents (PCI)" (in en). https://www.bhf.org.uk/informationsupport/treatments/coronary-angioplasty-and-stents.
- ↑ "Stents - What to Expect After Getting a Stent | NHLBI, NIH" (in en). 24 March 2022. https://www.nhlbi.nih.gov/health/stents/recovery.
- ↑ "Coronary angioplasty and stent insertion - Recovery" (in en). 11 June 2018. https://www.nhs.uk/conditions/coronary-angioplasty/recovery/.
- ↑ "Discharge advice after your coronary angiogram, angioplasty or stent insertion (PCI)" (in en-GB). 9 April 2021. https://www.hey.nhs.uk/patient-leaflet/discharge-advice-after-your-coronary-angiogram-angioplasty-or-stent-insertion-pci/.
- ↑ "Stents - Living With a Stent | NHLBI, NIH" (in en). 24 March 2022. https://www.nhlbi.nih.gov/health/stents/living-with.
- ↑ "How Cardiac Rehabilitation Can Help Heal Your Heart" (in en-us). U.S. Centers for Disease Control and Prevention. 12 September 2022. https://www.cdc.gov/heartdisease/cardiac_rehabilitation.htm.
- ↑ "In-stent restenosis in the drug-eluting stent era". Journal of the American College of Cardiology 56 (23): 1897–1907. November 2010. doi:10.1016/j.jacc.2010.07.028. PMID 21109112.
- ↑ 120.0 120.1 "Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease". The New England Journal of Medicine 375 (13): 1242–1252. September 2016. doi:10.1056/NEJMoa1607991. PMID 27572953.
- ↑ "Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review". Circulation: Cardiovascular Interventions 12 (8). August 2019. doi:10.1161/CIRCINTERVENTIONS.118.007023. PMID 31345066.
- ↑ 122.0 122.1 "Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis". Journal of the American College of Cardiology 65 (23): 2496–2507. June 2015. doi:10.1016/j.jacc.2015.04.017. PMID 26065988.
- ↑ 123.0 123.1 123.2 "Advances in stent technologies and their effect on clinical efficacy and safety". Medical Devices: Evidence and Research 7: 165–178. 2014. doi:10.2147/MDER.S31869. PMID 24940085.
- ↑ 124.0 124.1 124.2 "Mechanical Testing of Vascular Stents". ADMET. 29 November 2019. https://www.admet.com/blog/mechanical-testing-vascular-stents/.
- ↑ "Overview of Medical Device Classification and Reclassification". FDA. 3 November 2018. https://www.fda.gov/about-fda/cdrh-transparency/overview-medical-device-classification-and-reclassification. Retrieved 19 November 2023.
- ↑ Head, S. J.; Milojevic, M.; Daemen, J.; Ahn, J. M.; Boersma, E.; Christiansen, E. H.; Domanski, M. J.; Farkouh, M. E. et al. (2018). "Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: A pooled analysis of individual patient data". Lancet 391 (10124): 939–948. doi:10.1016/S0140-6736(18)30423-9. PMID 29478841. https://ueaeprints.uea.ac.uk/id/eprint/66412/1/Accepted_manuscript.pdf.
- ↑ "Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty". The New England Journal of Medicine 301 (2): 61–68. July 1979. doi:10.1056/NEJM197907123010201. PMID 449946.
- ↑ 128.0 128.1 128.2 128.3 "Percutaneous Coronary Revascularization". Harrison's Principles of Internal Medicine (16th ed.). New York: McGraw-Hill. 2005. pp. 1459–1462.
- ↑ "Transluminal Treatment of Arteriosclerotic Obstruction. Description of a New Technic and a Preliminary Report of Its Application". Circulation 30 (5): 654–670. November 1964. doi:10.1161/01.CIR.30.5.654. PMID 14226164.
- ↑ 130.0 130.1 130.2 130.3 130.4 "Coronary-artery stents". The New England Journal of Medicine 354 (5): 483–495. February 2006. doi:10.1056/NEJMra051091. PMID 16452560.
- ↑ "Physiological transport forces govern drug distribution for stent-based delivery". Circulation 104 (5): 600–605. July 2001. doi:10.1161/hc3101.092214. PMID 11479260.
- ↑ Strategic Approaches in Coronary Intervention. Lippincott Williams & Wilkins. 2006. ISBN 978-0-7817-4294-8. https://books.google.com/books?id=uqft1t92S88C&q=stent+restenosis&pg=PA358. Retrieved 13 May 2015.
- ↑ "Guideline on the development of medicinal substances contained in drug-eluting (medicinal substance-eluting) coronary stents". London: European Medicines Agency. 22 March 2007. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting-coronary-stents_en.pdf.
- ↑ 134.0 134.1 134.2 "Sirolimus: a new immunosuppressant". J Assoc Physicians India 53: 885–90. October 2005. PMID 16459533.
- ↑ Sirolimus-Eluting Stents for Chronic Total Coronary Occlusions - Full Text View - ClinicalTrials.gov. 5 March 2007. https://clinicaltrials.gov/ct2/show/NCT00258596. Retrieved 8 February 2024.
- ↑ Sirolimus-eluting Stents With Biodegradable Polymer Versus an Everolimus-eluting Stents - Full Text View - ClinicalTrials.gov. 16 October 2018. https://clinicaltrials.gov/ct2/show/NCT01443104. Retrieved 8 February 2024.
- ↑ Clinical Trial on the Efficacy and Safety of Sirolimus-Eluting Stent (MiStent® System) - Full Text View - ClinicalTrials.gov. 3 September 2020. https://clinicaltrials.gov/ct2/show/NCT02448524. Retrieved 8 February 2024.
- ↑ Mauri, Laura; Hsieh, Wen-hua; Massaro, Joseph M.; Ho, Kalon K.L.; D'Agostino, Ralph; Cutlip, Donald E. (8 March 2007). "Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents". New England Journal of Medicine 356 (10): 1020–1029. doi:10.1056/NEJMoa067731. PMID 17296821. http://www.nejm.org/doi/10.1056/NEJMoa067731. Retrieved 8 February 2024.
- ↑ A Randomized, Double-blind Study to Assess Paclitaxel-eluting Stents in Treatment of Longer Lesions - Full Text View - ClinicalTrials.gov. 20 April 2017. https://clinicaltrials.gov/ct2/show/NCT00297804. Retrieved 8 February 2024.
- ↑ Dawkins, Keith D.; Grube, Eberhard; Guagliumi, Giulio; Banning, Adrian P.; Zmudka, Krzysztof; Colombo, Antonio; Thuesen, Leif; Hauptman, Karl et al. (22 November 2005). "Clinical Efficacy of Polymer-Based Paclitaxel-Eluting Stents in the Treatment of Complex, Long Coronary Artery Lesions From a Multicenter, Randomized Trial: Support for the Use of Drug-Eluting Stents in Contemporary Clinical Practice". Circulation 112 (21): 3306–3313. doi:10.1161/CIRCULATIONAHA.105.552190. PMID 16286586. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.552190. Retrieved 8 February 2024.
- ↑ "Clinical Trial Results - Eluvia Drug-Eluting Stent". https://www.bostonscientific.com/en-EU/medical-specialties/vascular-surgery/drug-eluting-therapies1/eluvia/eluvia-clinical-trials.html.
- ↑ Galløe, Anders M.; Thuesen, Leif; Kelbæk, Henning; Thayssen, Per; Rasmussen, Klaus; Hansen, Peter R.; Bligaard, Niels; Saunamäki, Kari et al. (30 January 2008). "Comparison of Paclitaxel- and Sirolimus-Eluting Stents in Everyday Clinical PracticeThe SORT OUT II Randomized Trial". JAMA 299 (4): 409–416. doi:10.1001/jama.299.4.409. PMID 18230778.
- ↑ Dai, Yi; Wang, Rutao; Chen, Fengying; Zhang, Yaojun; Liu, Yi; Huang, He; Yang, Ping; Zhang, Ruining et al. (12 November 2021). "Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry". BMC Cardiovascular Disorders 21 (1): 537. doi:10.1186/s12872-021-02356-0. PMID 34772347.
- ↑ "First randomised controlled trial comparing the sirolimus-eluting bioadaptor with the zotarolimus-eluting drug-eluting stent in patients with de novo coronary artery lesions: 12-month clinical and imaging data from the multi-centre, international, BIODAPTOR-RCT". eClinicalMedicine 65. November 2023. doi:10.1016/j.eclinm.2023.102304. PMID 38106564. PMC 10725075. https://www.thelancet.com/pdfs/journals/eclinm/PIIS2589-5370%2823%2900481-9.pdf. Retrieved 8 February 2024.
- ↑ Commissioner, Office of the (3 November 2018). "Jurisdictional Update: Drug-Eluting Cardiovascular Stents". FDA. https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-drug-eluting-cardiovascular-stents. Retrieved 8 February 2024.
- ↑ 146.0 146.1 "Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation". Biotechnology Advances 36 (6): 1608–1621. November 2018. doi:10.1016/j.biotechadv.2018.04.002. PMID 29678389.
- ↑ "Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials - The Lancet". The Lancet 393 (10190): 2503–2510. June 2019. doi:10.1016/S0140-6736(19)30474-X. PMID 31056295. https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736%2819%2930474-X.pdf. Retrieved 8 February 2024.
- ↑ Li, Mingxuan; Tu, Haixia; Yan, Yu; Guo, Zhen; Zhu, Haitao; Niu, Jiangliang; Yin, Mengchen (21 September 2023). "Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries". PLOS ONE 18 (9). doi:10.1371/journal.pone.0291466. PMID 37733656. Bibcode: 2023PLoSO..1891466L.
- ↑ "Frequency and Predictors of Drug-Eluting Stent Use in Saphenous Vein Bypass Graft Percutaneous Coronary Interventions". JACC: Cardiovascular Interventions 3 (10): 1068–1073. 2010. doi:10.1016/j.jcin.2010.07.009. PMID 20965466.
- ↑ "Acceptance, Panic, and Partial Recovery". JACC: Cardiovascular Interventions 3 (9): 902–910. 2010. doi:10.1016/j.jcin.2010.06.014. PMID 20850088.
- ↑ 151.0 151.1 "Magnesium-Based Bioresorbable Stent Materials: Review of Reviews". Journal of Bio- and Tribo-Corrosion 5 (1). 2019. doi:10.1007/s40735-019-0216-x. Bibcode: 2019JBTC....5...26A. https://link.springer.com/article/10.1007/s40735-019-0216-x. Retrieved 11 March 2024.
- ↑ "Biodegradable Metals". Degradation of Implant Materials. Springer. 2012. pp. 93–109. doi:10.1007/978-1-4614-3942-4_5. ISBN 978-1-4614-3941-7. https://link.springer.com/chapter/10.1007/978-1-4614-3942-4_5. Retrieved 11 March 2024.
- ↑ 153.0 153.1 153.2 "Bioabsorbable coronary stents". Circulation: Cardiovascular Interventions 2 (3): 255–260. June 2009. doi:10.1161/CIRCINTERVENTIONS.109.859173. PMID 20031723.
- ↑ 154.0 154.1 "Fully bioresorbable drug-eluting coronary scaffolds: A review". Archives of Cardiovascular Diseases 108 (6–7): 385–397. 2015. doi:10.1016/j.acvd.2015.03.009. PMID 26113479.
- ↑ Damian Garde for Fierce Medical Devices. 22 May 2013 Boston Scientific, Elixir make waves at EuroPCR 2013
- ↑ Ni, Le; Chen, Hao; Luo, Zhurong; Yu, Yunqiang (28 January 2020). "Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis". Journal of Cardiothoracic Surgery 15 (1): 26. doi:10.1186/s13019-020-1041-5. PMID 31992360.
- ↑ "Abbott Pulls Troubled Absorb Stent From European Market". 6 April 2017. http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/.
- ↑ "Boston Scientific to end Renuvia bioresorbable coronary stent program – MassDevice". +MassDevice. 31 July 2017. http://www.massdevice.com/boston-scientific-end-renuvia-bioresorbable-coronary-stent-program/.
- ↑ 159.0 159.1 "The Current Literature on Bioabsorbable Stents: A Review". Current Atherosclerosis Reports 21 (12). 2019. doi:10.1007/s11883-019-0816-4. PMID 31768641. https://link.springer.com/content/pdf/10.1007/s11883-019-0816-4.pdf. Retrieved 11 March 2024.
- ↑ "Advancing Toward 3D Printing of Bioresorbable Shape Memory Polymer Stents". Biomacromolecules 21 (10): 3957–3965. 2020. doi:10.1021/acs.biomac.0c01082. PMID 32924443.
- ↑ Kirillova, Alina; Yeazel, Taylor R.; Asheghali, Darya; Petersen, Shannon R.; Dort, Sophia; Gall, Ken; Becker, Matthew L. (22 September 2021). "Fabrication of Biomedical Scaffolds Using Biodegradable Polymers". Chemical Reviews 121 (18): 11238–11304. doi:10.1021/acs.chemrev.0c01200. PMID 33856196. https://pubs.acs.org/doi/10.1021/acs.chemrev.0c01200. Retrieved 13 March 2024.
- ↑ "Abbott Restarts Bioresorbable Stent Clinical Trials with New Esprit Below-the-knee Scaffold". 3 September 2020. https://www.dicardiology.com/article/abbott-restarts-bioresorbable-stent-clinical-trials-new-esprit-below-knee-scaffold.
- ↑ "Bioresorbable vascular scaffolds versus conventional drug-eluting stents across time: A meta-analysis of randomised controlled trials". Open Heart 9 (2). 2022. doi:10.1136/openhrt-2022-002107. PMID 36288820.
- ↑ "Fully Bioresorbable Vascular Stents". Current Trends in Biomedical Engineering. Springer. 2023. pp. 255–268. doi:10.1007/978-3-031-38743-2_14. ISBN 978-3-031-38742-5. https://link.springer.com/chapter/10.1007/978-3-031-38743-2_14. Retrieved 11 March 2024.
- ↑ "TCT-841 Baseline characteristics and 3-month outcomes of the EVOLVE Short DAPT Trial: A prospective investigation of abbreviated antiplatelet therapy in high bleeding risk patients treated with a thin-strut bioabsorbable polymer-coated, everolimus-eluting coronary stent" (in en). Journal of the American College of Cardiology 72 (13 Supplement): B335–B336. 22 September 2018. doi:10.1016/j.jacc.2018.08.2086. ISSN 0735-1097.
- ↑ "Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET All Comers): a multicentre, open-label, randomised non-inferiority trial". Lancet 392 (10153): 1117–1126. September 2018. doi:10.1016/S0140-6736(18)31649-0. PMID 30190206.
- ↑ "The ABSORB bioresorbable vascular scaffold: an evolution or revolution in interventional cardiology?". Hellenic Journal of Cardiology 53 (4): 301–309. 2012. PMID 22796817. http://www.hellenicjcardiol.org/archive/full_text/2012/4/2012_4_301.pdf. Retrieved 13 January 2016.
- ↑ 168.0 168.1 168.2 168.3 "Contemporary coronary drug-eluting and coated stents: an updated mini-review (2023)". Cardiovascular Intervention and Therapeutics 39 (1): 15–17. September 2023. doi:10.1007/s12928-023-00954-7. PMID 37656338.
- ↑ "Drug-Eluting Stents Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028". Research and Markets Ltd. https://www.researchandmarkets.com/reports/5732651/drug-eluting-stents-market-global-industry.
- ↑ "Drug-eluting Stent Market Size, share & Growth Research Report". https://www.bccresearch.com/market-research/medical-devices-and-surgical/drug-eluting-stent-market.html.
- ↑ "Evolution of drug-eluting coronary stents: a back-and-forth journey from the bench to bedside". Cardiovascular Research 119 (3): 631–646. May 2023. doi:10.1093/cvr/cvac105. PMID 35788828.
- ↑ "Health economic evaluation of the use of drug-eluting stents: First results from the Drug-Eluting Stent Registry (DES.de)". Herz 38 (1): 57–64. February 2012. doi:10.1007/s00059-012-3581-5. PMID 22301731.
- ↑ "Economic evaluation of drug-eluting stents: a systematic literature review and model-based cost-utility analysis". International Journal of Technology Assessment in Health Care 23 (4): 473–479. 2007. doi:10.1017/S0266462307070560. PMID 17937836.
- ↑ "Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials". Archives of Internal Medicine 172 (4): 312–319. February 2012. doi:10.1001/archinternmed.2011.1484. PMID 22371919.
- ↑ "No Extra Benefits Are Seen in Stents for Coronary Artery Disease" (in en-US). The New York Times. 27 February 2012. ISSN 0362-4331. https://www.nytimes.com/2012/02/28/health/stents-show-no-extra-benefits-for-coronary-artery-disease.html.
- ↑ "Surgery for Blocked Arteries Is Often Unwarranted, Researchers Find" (in en-US). The New York Times. 16 November 2019. ISSN 0362-4331. https://www.nytimes.com/2019/11/16/health/heart-disease-stents-bypass.html.
- ↑ An introduction to the US health care industry: balancing care, cost, and access. Baltimore: Johns Hopkins University Press. 2020. ISBN 978-1-4214-3882-5.
- ↑ "To Stent or Not to Stent?" (in en-US). Clinical Correlations. New York University (NYU) Department of Medicine. 6 November 2013. https://www.clinicalcorrelations.org/2013/11/06/to-stent-or-not-to-stent/.
- ↑ "Variation in patients' perceptions of elective percutaneous coronary intervention in stable coronary artery disease: cross sectional study". BMJ 349. September 2014. doi:10.1136/bmj.g5309. PMID 25200209.
- ↑ "Expert Answers on E.M.D.R." (in en). Consults Blog. The New York Times. 16 March 2012. https://archive.nytimes.com/consults.blogs.nytimes.com/2012/03/16/expert-answers-on-e-m-d-r/.
- ↑ "Patients Before Profits (1 Letter)" (in en-US). The New York Times. 5 March 2012. ISSN 0362-4331. https://www.nytimes.com/2012/03/06/health/patients-before-profits-1-letter.html.
- ↑ "Unnecessary stent usage worries doctors across India". Times of India. 30 January 2013. http://timesofindia.indiatimes.com/india/Unnecessary-stent-usage-worries-doctors-across-India/articleshow/18249217.cms.
- ↑ "In India, a call to halt financial incentives for stent use". Fierce Medical Devices. 30 January 2013. http://www.fiercemedicaldevices.com/story/india-call-halt-financial-incentives-stent-use/2013-01-30.
- ↑ "Profits from medical devices used to bribe doctors?". The Times of India. 15 September 2014. http://timesofindia.indiatimes.com/india/Profits-from-medical-devices-used-to-bribe-doctors/articleshow/42484806.cms.
- ↑ Neumann, F. J., et al. "2018 ESC/EACTS Guidelines on myocardial revascularization." European Heart Journal, vol. 40, no. 2, 2019, pp. 87–165. https://doi.org/10.1093/eurheartj/ehy394
- ↑ Bangalore, S., et al. "Percutaneous coronary intervention vs. optimal medical therapy in stable ischemic heart disease: Meta-analysis of randomized trials." Circulation, vol. 141, no. 16, 2020, pp. 132–143. https://doi.org/10.1161/CIRCULATIONAHA.119.044870
- ↑ 187.0 187.1 "Boston Scientific fined $42M in stent patent lawsuit". Fierce Biotech. 3 February 2023. https://www.fiercebiotech.com/medtech/boston-scientific-found-guilty-infringing-drug-eluting-stent-patent-fined-42m.
- ↑ 188.0 188.1 "Boston Scientific hit with $42M verdict in drug-eluting stents IP case". 2 February 2023. https://www.massdevice.com/boston-scientific-hit-with-42m-verdict-in-drug-eluting-stents-ip-case/.
- ↑ "Annual Review of Medical Device Patent Litigation | Publications | Insights". Faegre Drinker Biddle & Reath LLP. https://www.faegredrinker.com/en/insights/publications/2009/3/annual-review-of-medical-device-patent-litigation.
- ↑ 190.0 190.1 "Heart Stent Problems - Drug Eluting Stent Lawsuits". Saiontz & Kirk, P.A.. https://www.youhavealawyer.com/stent/heart-stent-problems/.
- ↑ 191.0 191.1 "Heart Stent Class Action Lawsuit". The Schmidt Firm, PLLC. https://www.schmidtlaw.com/heart-stent-class-action-lawsuit/.
 |
