Medicine:Fecal occult blood
| Fecal occult blood | |
|---|---|
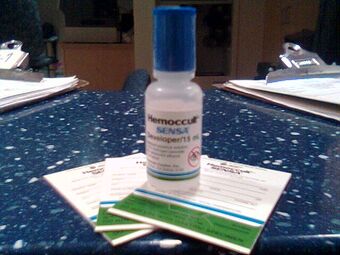 | |
| Cards and bottle used for the Hemoccult test, a type of stool guaiac test | |
| Specialty | Gastroenterology, general surgery |
Fecal occult blood (FOB) refers to blood in the feces that is not visibly apparent (unlike other types of blood in stool such as melena or hematochezia). A fecal occult blood test (FOBT) checks for hidden (occult) blood in the stool (feces).[1]
The American College of Gastroenterology has recommended the abandoning of gFOBT testing as a colorectal cancer screening tool, in favor of the fecal immunochemical test (FIT).[2] The newer and recommended tests look for globin, DNA, or other blood factors including transferrin, while conventional stool guaiac tests look for heme.
Medical uses
Fecal occult blood testing (FOBT), as its name implies, aims to detect subtle blood loss in the gastrointestinal tract, anywhere from the mouth to the colon. Positive tests ("positive stool") may result from either upper gastrointestinal bleeding or lower gastrointestinal bleeding and warrant further investigation for peptic ulcers or a malignancy (such as colorectal cancer or gastric cancer). The test does not directly detect colon cancer but is often used in clinical screening for that disease. It can also be used to look for active occult blood loss in anemia[3] or when there are gastrointestinal symptoms.[4]
Colorectal cancer screening
An estimated 1–5% of large tested populations have a positive fecal occult blood test.[citation needed] Of those, about 2–10% have cancer, while 20–30% have adenomas. Screening methods for colon cancer depend on detecting either precancerous changes such as certain kinds of polyps or on finding early and thus more treatable cancer. The extent to which screening procedures reduce the risk of gastrointestinal cancer or deaths depends on the rate of precancerous and cancerous disease in that population. gFOBT (guaiac fecal occult blood test) and flexible sigmoidoscopy screening have each shown benefit. Other colon cancer screening tools such as iFOBT (immunochemical fecal occult blood test) or colonoscopy are also included in guidelines.[5]
In 2009, the American College of Gastroenterology (ACG) suggested that colon cancer screening modalities that are also directly preventive by removing precursor lesions should be given precedence, and prefer a colonoscopy every ten years in average-risk individuals, beginning at age 50.[6] The ACG suggests that cancer detection tests such as any type of FOB are an alternative that is less preferred, and if a colonoscopy is declined, the FIT (fecal immunochemical test, or iFOBT) should be offered instead. The 2017 US Multi-Society Task Force (MSTF)'s recommended first-tier tests are a colonoscopy every 10 years or annual FIT test.[7] If FIT is utilized, proper steps must be taken to ensure appropriate use and follow-up of abnormal FIT results.[8] FIT tests however are not that useful in picking up adenomas, even when advanced.[9]
The United States Preventive Services Task Force (USPSTF)'s 2016 recommendation, instead of emphasizing specific screening approaches, has instead chosen to highlight that there is convincing evidence that colorectal cancer screening substantially reduces deaths from the disease among adults aged 50 to 75 years and that not enough adults are using this effective preventive intervention.[10] The ACG and MSTF also included CT colonography every five years, and fecal DNA testing as considerations. All three recommendation panels recommended replacing any older low-sensitivity, guaiac-based fecal occult blood testing (gFOBT) with either newer high-sensitivity guaiac-based fecal occult blood testing (hs gFOBT) or fecal immunochemical testing (FIT). MSTF looked at six studies that compared high-sensitivity gFOBT (Hemoccult SENSA) to FIT, and concluded that there was no clear difference in overall performance between these methods.
The English National Health Service (NHS) introduced a Bowel Cancer Screening Program in 2006.[11] It is now offered to patients aged 60–74 years. In 2019 FIT was introduced as the primary screening test in England and Wales, replacing gFOBt. [12][13] However, research carried out in the UK has suggested that the FIT threshold for further investigation is set at a point that may miss more than half of bowel cancer cases and only identifies one in four high-risk polyps.[14][15]
The American College of Gastroenterology has recommended the abandoning of gFOBT testing as a colorectal cancer screening tool, in favor of the fecal immunochemical test.[6] Though the FIT test is preferred, even the guaiac FOB testing of average risk populations may have been sufficient to reduce the mortality associated with colon cancer by about 25%.[16] With this lower efficacy, it was not always cost-effective to screen a large population with gFOBT.[17][18][19][20]
If colon cancer is suspected in an individual (such as in someone with an unexplained anemia), fecal occult blood tests may not be clinically helpful. If a doctor suspects colon cancer, more rigorous investigation is necessary, whether or not the test is positive.[citation needed]
In 2006, the Australian Government introduced the National Bowel Cancer Program which has been updated several times since; targeted screening will be done of all Australians aged from 50 to 74 by 2020. Cancer Council Australia recommended that FOBT should be done every two years. People over 50 not yet eligible for the national program can arrange with their doctor for an FOBT.[21] The Canadian Cancer Society recommends that men and women aged 50 and over have an FOBT at least every two years.[22] In colon cancer screening, using only one sample of feces collected by a doctor performing a digital rectal examination is discouraged.[23]
The use of the M2-PK Test is encouraged over gFOBT for routine screening, as it may pick up tumors whether or not they are bleeding.[24] It is able to detect 80 percent of colorectal cancers and 44 percent for adenoma > 1 centimeter, while gFOBT picks up 13 to 50 percent of colorectal cancers.[24]
Other sources of bleeding
Gastrointestinal bleeding has many potential sources, and positive results usually result in further testing for the bleeding site, usually looking for lower gastrointestinal bleeding before upper gastrointestinal bleeding causes unless there are other clues.[25] Colonoscopy is usually preferred to computerized tomographic colonography.[26]
A positive test can result from upper gastrointestinal bleeding or lower gastrointestinal bleeding. The common causes are:
- 2–10%: cancer (colorectal cancer, gastric cancer)
- 20–30% adenoma or polyps
- Diverticular disease[27]
- Hemorrhoids[27]
- Inflammatory bowel disease[27]
- Angiodysplasia of the colon
- Sickle cell anemia
In the event of a positive fecal occult blood test, the next step in the workup is a form of visualization of the gastrointestinal tract by one of several means:
- Sigmoidoscopy, an examination of the rectum and lower colon with a lighted instrument to look for abnormalities, such as polyps.
- Colonoscopy, a more thorough examination of the rectum and entire colon.
- Virtual colonoscopy
- Upper gastrointestinal endoscopy. It is sometimes performed with chromoendoscopy, a method that assists the endoscopist by enhancing the visual difference between cancerous and normal tissue, by either marking the abnormally increased DNA content (toluidine blue) or failing to stain the tumor, possibly due to decreased surface glycogen on tumor cells(Lugol).[28][29] Infrared fluorescent endoscopy[citation needed] and ultrasonic endoscopy[citation needed] can interrogate vascular abnormalities such as esophageal varices.
- Double-contrast barium enema: a series of x-rays of the colon and rectum.
Testing secretions for blood
The use of an FOBT for bleeding from the mouth, nose, esophagus, lungs, stomach and the initial portion of the small intestine, while the same as fecal testing, is discouraged, due to technical considerations including poorly characterized test performance characteristics such as sensitivity, specificity, and analytical interference.[30] However, chemical confirmation that coloration is due to blood rather than coffee, beets, medications, or food additives can be of significant clinical assistance.
Marathon runners
Gastrointestinal (GI) complaints and low-intensity GI bleeding frequently occur in marathon runners.[31] Strenuous exercise, particularly in elite athlete runners and less frequently in other exercise activities, can cause acute incapacitating gastrointestinal symptoms including heartburn, nausea, vomiting, abdominal pain, diarrhea and gastrointestinal bleeding.[32] Approximately one third of endurance runners experience transient but exercise-limiting symptoms, and repetitive gastrointestinal bleeding occasionally causes iron deficiency and anaemia.[33][34] Runners can sometimes experience significant symptoms including hematemesis.[35] Exercise is associated with extensive changes in gastrointestinal (GI) tract physiology, including diversion of blood flow from the GI tract to muscles and lungs, decreased GI absorption and small intestinal motility, increased colonic transit, neuroimmunoendocrine changes in hormones and peptides such as vasoactive intestinal peptide, secretin and peptide-histidine-methionine.[36] Substantial changes occur in stress hormones including cortisol, in circulating concentrations and metabolic behavior of various leucocytes, and in immunoglobulin levels and major histocompatibility complex expression.[37] Symptoms can be exacerbated by dehydration or by pre-exercise ingestion of certain foods and hypertonic liquids, and lessened by adequate training.[36]
Ingestion of 800 mg of cimetidine two hours before running a marathon did not significantly affect the frequency of gastrointestinal symptoms or occult gastrointestinal bleeding.[38] Conversely, 800 mg of cimetidine 1 hr before the start and again at 50 miles of a 100-mile running race substantially decreased GI symptoms and post-race guaiac test positivity but did not affect race performance.[39]
Methodology
There are four methods in clinical use to test for occult blood in feces. These look at different properties, such as antibodies, heme, globin, or porphyrins in blood, or at DNA from cellular material such as from lesions of the intestinal mucosa.
- Fecal immunochemical testing (FIT), and immunochemical fecal occult blood test (iFOBT). FIT products utilize specific antibodies to detect globin. FIT screening is more effective in terms of health outcomes and cost compared with guaiac FOBT.[40] According to the guidelines of the American College of Gastroenterology, "Annual fecal immunochemical testing is the preferred colorectal cancer detection test."[6][41] A FIT test detects globin levels in feces at or above 50 nanograms per mL, the established cutoff by the World Health Organization for Colorectal Cancer Screening.[citation needed] FIT testing has replaced most gFOBT tests as the colon cancer screening test of choice.[42][43] This methodology can be adapted for automated test reading and to report quantitative results, which are potential factors in design of a widescale screening strategy.[44] The number of fecal samples submitted for FIT may affect the clinical sensitivity and specificity of the methodology.[8] High-sensitivity gFOBT tests such as Hemoccult SENSA remain an accepted option[8] and may retain a role in monitoring gastrointestinal conditions such as ulcerative colitis;[45] however, the FIT test is preferred in recent guidelines.[6] FIT is widely used outside of the US, and generally cost less than US$20 per test in 2020, compared to US$1,000 or more for a colonoscopy.[46]
- Stool guaiac test for fecal occult blood (gFOBT): – The stool guaiac test involves smearing some feces onto some absorbent paper that has been treated with a chemical. Hydrogen peroxide is then dropped onto the paper; if trace amounts of blood are present, the paper will change color in one or two seconds. This method works as the heme component in hemoglobin has a peroxidase-like effect, rapidly breaking down hydrogen peroxide. In some settings such as gastric or proximal upper intestinal bleeding, the guaiac method may be more sensitive than tests detecting globin because globin is broken down in the upper intestine to a greater extent than is heme.[47] There are various commercially available gFOBT tests which have been categorized as being of low or high sensitivity, and only high-sensitivity tests remain an acceptable alternative to FIT testing, which is now the best-practices recommendation in colon cancer screening. Optimal clinical performance of the stool guaiac test depends on preparatory dietary adjustment.[48] The stool guaiac test for hidden (occult) blood in the stool should be used at home following the test kit's directions with spontaneously passed stool[23] or on samples submitted to a clinical laboratory. Testing kits are available at pharmacies in some countries without a prescription, or a health professional may order a testing kit for use at home. If a home fecal occult blood test detects blood in the stool it is recommended to see a health professional to arrange further testing.[49]
- Stool DNA screening tests look for DNA alterations that have been associated with cancer.
Additional methods of looking for occult blood are being explored, including transferrin dipstick[50] and stool cytology.[51]
Test performance
Reference standards
The estimates for test performance characteristics are based on comparison with a variety of reference methods including 51-chromium studies,[citation needed] analytical recovery studies in spiked stool samples, analytical recovery after ingestion of autologous blood, rarer studies of carefully quantified blood instilled at bowel surgery [citation needed], as well as other research approaches.[citation needed] Additionally, clinical studies look at a variety of additional factors.
Gastrointestinal blood loss
In healthy people about 0.5 to 1.5 ml of blood escapes blood vessels into the stool each day.[52][53][54] Significant amounts of blood can be lost without producing visible blood in the stool, estimated as 200 ml in the stomach,[55] 100 ml in the duodenum, and lesser amounts in the lower intestine. Tests for occult blood identify lesser blood loss.
Clinical sensitivity and specificity
Fecal immunochemical testing (FIT) can identify as little as 0.3 ml of daily blood in the stool; yet this test threshold does not cause undue false positives from normal upper intestinal blood leakage because it does not detect occult blood from the stomach and upper small intestine. Thus, the FIT test is much more specific for bleeding from the colon or lower gastrointestinal tract than alternatives.[56] The detection rate of the test decreases if the time from sample collection to laboratory processing is delayed; processing the sample in under five days from collection is recommended.[57] It does not appear to be affected by aspirin, anticoagulants, or nonsteroidal anti-inflammatory drugs.[58]
Stool guaiac test for fecal occult blood (gFOBT) sensitivity varies depending on the site of bleeding. Moderately sensitive gFOBT can pick up a daily blood loss of about 10 ml (about two teaspoonfuls), and higher sensitivity gFOBT can pick up lesser amounts, requires at least 2 ml to become positive. The sensitivity of a single-stool guaiac test to pick up bleeding has been quoted at 10 to 30%, but if a standard three tests are done as recommended the sensitivity rises to 92%.[59] Reduced patient compliance with the collection of three samples hampers the usefulness of this test. Further discussion of sensitivity and specificity issues that relate particularly to the guaiac method is found in the stool guaiac test article.
Fecal porphyrin quantification by HemoQuant can yield a false positive result due to exogenous blood and various porphyrins. HemoQuant is the most sensitive test for upper gastrointestinal bleeding and therefore may be most appropriate fecal occult blood test to use in the evaluation of iron deficiency.[60] It is advisable to stop ingesting red meat and aspirin for three days prior to specimen collection.[61] False positives can occur with myoglobin, catalase, or protohemes[62] and in certain types of porphyria.[citation needed]
Fecal DNA tests as of 2008 had not been studied enough to support widespread use.[63]
Regulation
Safety regulations from US accreditor the Joint Commission may have unintentionally decreased digital rectal examination and FOBT in hospital settings such as Emergency Departments.[64][65]
References
- ↑ "Occult Gastrointestinal Bleeding: Detection, Interpretation, and Evaluation". JIACM 3 (2): 153–58. 2002. http://medind.nic.in/jac/t02/i2/jact02i2p153.pdf.
- ↑ "American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]". The American Journal of Gastroenterology 104 (3): 739–750. March 2009. doi:10.1038/ajg.2009.104. PMID 19240699.
- ↑ "Fecal occult blood testing for iron deficiency: a reappraisal". Digestive Diseases 18 (2): 75–82. 2000. doi:10.1159/000016968. PMID 11060470.
- ↑ "Screening of patients with acute infectious diarrhoea: evaluation of clinical features, faecal microscopy, and faecal occult blood testing". Scandinavian Journal of Gastroenterology 35 (1): 54–60. January 2000. doi:10.1080/003655200750024533. PMID 10672835.
- ↑ "Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer". Gastroenterology 152 (5): 1217–1237.e3. April 2017. doi:10.1053/j.gastro.2016.08.053. PMID 27769517.
- ↑ 6.0 6.1 6.2 6.3 "American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]". The American Journal of Gastroenterology 104 (3): 739–750. March 2009. doi:10.1038/ajg.2009.104. PMID 19240699.
- ↑ "Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer". Gastroenterology (Elsevier BV) 153 (1): 307–323. July 2017. doi:10.1053/j.gastro.2017.05.013. PMID 28600072.
- ↑ "Making FIT Count: Maximizing Appropriate Use of the Fecal Immunochemical Test for Colorectal Cancer Screening Programs". Journal of General Internal Medicine (JGIM) 35 (6): 1870–1874. June 2020. doi:10.1007/s11606-020-05728-y. PMID 32128688.
- ↑ "Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis". Annals of Internal Medicine 170 (5): 319–329. March 2019. doi:10.7326/M18-2390. PMID 30802902.
- ↑ "Final Recommendation Statement: Colorectal Cancer: Screening". U.S. Preventive Services Task Force. June 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2.
- ↑ "The NHS Bowel Cancer Screening Program: current perspectives on strategies for improvement" (in English). Risk Management and Healthcare Policy 10: 177–187. 2017-12-04. doi:10.2147/RMHP.S109116. PMID 29270036.
- ↑ "CKS is only available in the UK". https://www.nice.org.uk/cks-uk-only.
- ↑ "Bowel cancer screening" (in en). 2017-10-20. https://www.nhs.uk/conditions/bowel-cancer-screening/.
- ↑ (in en-GB) New pathways could improve bowel cancer screening. 2021-09-13. doi:10.3310/alert_47581. https://evidence.nihr.ac.uk/alert/new-pathways-could-improve-bowel-screening-programme/. Retrieved 2022-08-05.
- ↑ "Faecal immunochemical testing in bowel cancer screening: Estimating outcomes for different diagnostic policies". Journal of Medical Screening 28 (3): 277–285. September 2021. doi:10.1177/0969141320980501. PMID 33342370.
- ↑ "Evidence for colorectal cancer screening". Best Practice & Research. Clinical Gastroenterology 24 (4): 417–425. August 2010. doi:10.1016/j.bpg.2010.06.005. PMID 20833346.
- ↑ "Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study". The New England Journal of Medicine 328 (19): 1365–1371. May 1993. doi:10.1056/NEJM199305133281901. PMID 8474513.
- ↑ "Randomised controlled trial of faecal-occult-blood screening for colorectal cancer". Lancet 348 (9040): 1472–1477. November 1996. doi:10.1016/S0140-6736(96)03386-7. PMID 8942775.
- ↑ "Randomised study of screening for colorectal cancer with faecal-occult-blood test". Lancet 348 (9040): 1467–1471. November 1996. doi:10.1016/S0140-6736(96)03430-7. PMID 8942774.
- ↑ "Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects". Scandinavian Journal of Gastroenterology 29 (5): 468–473. May 1994. doi:10.3109/00365529409096840. PMID 8036464.
- ↑ "Bowel cancer screening program". 19 June 2018. https://www.cancer.org.au/about-cancer/early-detection/screening-programs/bowel-cancer-screening/.
- ↑ "Screening for colorectal cancer". 2018. http://www.cancer.ca/en/cancer-information/cancer-type/colorectal/screening.
- ↑ 23.0 23.1 "Accuracy of screening for fecal occult blood on a single stool sample obtained by digital rectal examination: a comparison with recommended sampling practice". Annals of Internal Medicine 142 (2): 81–85. January 2005. doi:10.7326/0003-4819-142-2-200501180-00006. PMID 15657155.
- ↑ 24.0 24.1 "Faecal pyruvate kinase isoenzyme type M2 for colorectal cancer screening: a meta-analysis". World Journal of Gastroenterology 18 (30): 4004–4011. August 2012. doi:10.3748/wjg.v18.i30.4004. PMID 22912551.
- ↑ "Occult gastrointestinal bleeding". Gastroenterology Clinics of North America 34 (4): 699–718. December 2005. doi:10.1016/j.gtc.2005.08.010. PMID 16303578.
- ↑ "What is the value of computered tomography colonography in patients screening positive for fecal occult blood? A systematic review and economic evaluation". Clinical Gastroenterology and Hepatology 5 (12): 1439–46; quiz 1368. December 2007. doi:10.1016/j.cgh.2007.09.003. PMID 18054752.
- ↑ 27.0 27.1 27.2 "Non-neoplastic findings at colonoscopy after positive faecal occult blood testing: data from the English Bowel Cancer Screening Programme". Journal of Medical Screening 21 (2): 89–94. June 2014. doi:10.1177/0969141314528889. PMID 24644029.
- ↑ "Observation of esophageal lesions with the use of endoscopic dyes.". Progress of Digestive Endoscopy 1: 34. 1972.
- ↑ "How can we diagnose the early stage of esophageal cancer? Endoscopic diagnosis". Endoscopy 18 (Suppl 3): 11–18. September 1986. doi:10.1055/s-2007-1018435. PMID 2428607.
- ↑ "Fecal Occult Blood Testing in Hospitalized Patients with Upper Gastrointestinal Bleeding". Journal of Hospital Medicine 12 (7): 567–569. July 2017. doi:10.12788/jhm.2773. PMID 28699947.
- ↑ "Gastrointestinal bleeding in marathon runners". Scandinavian Journal of Gastroenterology 21 (4): 493–497. May 1986. doi:10.3109/00365528609015168. PMID 3487825.
- ↑ "The impact of physical exercise on the gastrointestinal tract". Current Opinion in Clinical Nutrition and Metabolic Care 12 (5): 533–538. September 2009. doi:10.1097/MCO.0b013e32832e6776. PMID 19535976.
- ↑ "Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract". Gut 48 (3): 435–439. March 2001. doi:10.1136/gut.48.3.435. PMID 11171839.
- ↑ "Educational Case: Diverticulosis". Academic Pathology 9 (1): 100014. 2022-01-01. doi:10.1016/j.acpath.2022.100014. PMID 35600744.
- ↑ "Do some marathon runners bleed into the gut? (letter response to a previous article)". BMJ 288 (6410): 65. 1984. doi:10.1136/bmj.288.6410.65-b.
- ↑ 36.0 36.1 "Is the gut an athletic organ? Digestion, absorption and exercise". Sports Medicine 15 (4): 242–257. April 1993. doi:10.2165/00007256-199315040-00003. PMID 8460288.
- ↑ "Effect of dietary intake on immune function in athletes". Sports Medicine 32 (5): 323–337. 2002. doi:10.2165/00007256-200232050-00004. PMID 11929359.
- ↑ "Effect of cimetidine on marathon-associated gastrointestinal symptoms and bleeding". Digestive Diseases and Sciences 36 (10): 1390–1394. October 1991. doi:10.1007/BF01296804. PMID 1914760.
- ↑ "Cimetidine reduces running-associated gastrointestinal bleeding. A prospective observation". Digestive Diseases and Sciences 35 (8): 956–960. August 1990. doi:10.1007/BF01537243. PMID 2384041. https://zenodo.org/record/1232492.
- ↑ "[Chemical or immunological tests for the detection of fecal occult blood in colorectal cancer screening?]" (in es). Gastroenterologia y Hepatologia 32 (8): 565–576. October 2009. doi:10.1016/j.gastrohep.2009.01.179. PMID 19577340.
- ↑ Colorectal Cancer http://patients.gi.org/topics/colorectal-cancer
- ↑ "Which fecal occult blood test is best to screen for colorectal cancer?". Nature Clinical Practice. Gastroenterology & Hepatology 6 (3): 140–141. March 2009. doi:10.1038/ncpgasthep1358. PMID 19174764.
- ↑ "[Chemical or immunological tests for the detection of fecal occult blood in colorectal cancer screening?]" (in es). Gastroenterologia y Hepatologia 32 (8): 565–576. October 2009. doi:10.1016/j.gastrohep.2009.01.179. PMID 19577340.
- ↑ "Cost-effectiveness analysis of two strategies for mass screening for colorectal cancer in France". Health Economics 13 (3): 227–238. March 2004. doi:10.1002/hec.819. PMID 14981648.
- ↑ "Prediction of flare-ups of ulcerative colitis using quantitative immunochemical fecal occult blood test". World Journal of Gastroenterology 16 (9): 1110–1114. March 2010. doi:10.3748/wjg.v16.i9.1110. PMID 20205282.
- ↑ "A Colonoscopy Alternative Comes Home" (in en-US). The New York Times. 2021-01-11. ISSN 0362-4331. https://www.nytimes.com/2021/01/11/health/colonoscopy-health-home-testing.html.
- ↑ "Occult gastrointestinal bleeding". The New England Journal of Medicine 341 (1): 38–46. July 1999. doi:10.1056/NEJM199907013410107. PMID 10387941.
- ↑ "Diagnostic Tests — Fecal Occult Blood Test". http://www.health.harvard.edu/diagnostic-tests/fecal-occult-blood-test.htm.
- ↑ "Fecal Occult Blood Test (FOBT)". WebMD. http://www.webmd.com/colorectal-cancer/Fecal-Occult-Blood-Test-FOBT.
- ↑ "Transferrin dipstick as a potential novel test for colon cancer screening: a comparative study with immuno fecal occult blood test". Cancer Epidemiology, Biomarkers & Prevention 18 (8): 2182–2185. August 2009. doi:10.1158/1055-9965.EPI-09-0309. PMID 19661074.
- ↑ "Fecal cytology in conjunction with immunofecal occult blood test for colorectal cancer screening". Analytical and Quantitative Cytology and Histology 32 (3): 131–135. June 2010. PMID 20701065.
- ↑ "Fecal blood levels in health and disease. A study using HemoQuant". The New England Journal of Medicine 312 (22): 1422–1428. May 1985. doi:10.1056/NEJM198505303122204. PMID 3873009.
- ↑ "Occult faecal blood loss determined by chemical tests and a 51 Cr method". Scandinavian Journal of Gastroenterology 16 (2): 245–252. 1981. doi:10.3109/00365528109181963. PMID 7313535.
- ↑ "Evaluation of radiochromium blood loss studies in unexplained iron-deficiency anaemia". Australian and New Zealand Journal of Medicine 8 (2): 121–126. April 1978. doi:10.1111/j.1445-5994.1978.tb04496.x. PMID 307949.
- ↑ Schiff L, Stevens RJ, Shapiro N, et al. Observations on the oral administration of citrate blood in man. Am J Med Sci 1942; 203: 409.
- ↑ "What Does a Positive Fecal Occult Blood Test Mean? – B01". http://www.wdxcyber.com/ncanc10.htm.
- ↑ "False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening". International Journal of Cancer 125 (4): 746–750. August 2009. doi:10.1002/ijc.24458. PMID 19408302.
- ↑ "Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: a systematic review and meta-analysis". Gut 68 (5): 866–872. May 2019. doi:10.1136/gutjnl-2018-316344. PMID 29871970.
- ↑ "Screening average risk patients for colorectal cancer – B01". http://www.uptodate.com/.
- ↑ "Detection of occult upper gastrointestinal tract bleeding: performance differences in fecal occult blood tests". Mayo Clinic Proceedings 77 (1): 23–28. January 2002. doi:10.4065/77.1.23. PMID 11794453.
- ↑ "Test ID: HQ HemoQuant, Feces". Mayo Clinic: Mayo Clinical Laboratories. https://www.mayocliniclabs.com/test-catalog/overview/607706.
- ↑ "The "HemoQuant" test: a specific and quantitative determination of heme (hemoglobin) in feces and other materials". Clinical Chemistry 29 (12): 2061–2067. December 1983. doi:10.1093/clinchem/29.12.2061. PMID 6640900.
- ↑ "Screening for Colorectal Cancer: An Updated Systematic Review". Agency for Healthcare Research and Quality. 2008. Report No.: 08-05-05124-EF-1.. PMID 20722162.
- ↑ "The law of unintended consequences: The Joint Commission regulations and the digital rectal examination". Annals of Emergency Medicine 51 (2): 197–201, 201.e1. February 2008. doi:10.1016/j.annemergmed.2007.07.022. PMID 17961818. https://zenodo.org/record/1258732.
- ↑ "The effect of removal of point-of-care fecal occult blood testing on performance of digital rectal examinations in the emergency department". Annals of Emergency Medicine 56 (2): 135–141. August 2010. doi:10.1016/j.annemergmed.2009.12.021. PMID 20060198.
External links
- FOBT Overview at Mayo Clinic
- Overview at Cleveland Clinic
- ColonCancerCheck including fact sheets in 24 languages at Ontario Ministry of Health and Long-Term Care
- "Colorectal cancer screening". Current Opinion in Gastroenterology 26 (5): 466–470. September 2010. doi:10.1097/MOG.0b013e32833d1733. PMID 20664346.
| Classification | |
|---|---|
| External resources |
de:Guajak-Test
 |