Medicine:Laser-assisted drug delivery
Laser-assisted drug delivery (LADD) is a drug delivery technique commonly used in the dermatology field that involves lasers. As skin acts as a protective barrier to the environment, the absorption of topical products through the epidermis is limited; thus, different drug delivery modalities have been employed to improve the efficacy of these treatments. The use of lasers in LADD has been shown to enhance the penetration of drugs transdermal, leading to a higher absorption rate, limited systemic effects, and reduced duration of treatment. Although this technique has evolved in the past decade due to its efficacy through scientific research and clinical practice, there remain some limitations regarding the safety aspect that needs to be taken into consideration.
Transdermal drug delivery
The skin barrier
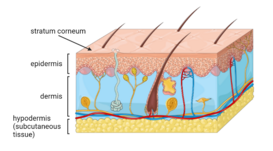
Skin is the largest organ in the human body that acts as the primary protective barrier against the external environment. It provides protection against ultraviolet light, trauma, pathogens, microorganisms, and toxins, sensory perception, temperature regulation, and immunity.[1] There are primarily three layers of skin, which include the outer epidermis, followed by the dermis and subcutaneous tissue, or hypodermis.[1] Skin is used as the target for drug delivery as it is a convenient route of drug administration, and the large area allows for different placements on the skin for transdermal delivery[1]
Transdermal delivery
Transdermal delivery is a non-invasive method commonly assisted in transporting topical products into intact and healthy skin. The substances initially penetrate through the stratum corneum, which is the outermost layer of the epidermis, then diffuse into the deeper epidermis and dermis layers for a systemic effect.[2] Although transdermal drug delivery presents several advantages as compared to other conventional modalities such as oral and parenteral routes,[2][3][4][5] the complexity of the skin barrier limits the methodology to reach its full potential.
Improvement in transdermal delivery
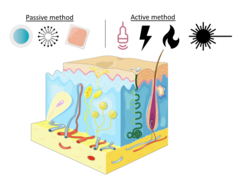
Various technologies have been developed to enhance the permeability of transdermal drugs, which can be divided into passive or chemical and active or physical methods. The passive approach involves the optimization of drug and vehicle interaction that could modify the stratum corneum structure or the addition of penetration enhancers for better absorption rates.[2] Some of the limitations of this approach include lag time in drug release, low efficiency, and skin irritation.[2] The active approach involves ultrasound, electrical stimulation, thermal approach, and mechanical approach.[2] These techniques facilitate the transportation of drugs by using energy as a driving force. Within the thermal approach, laser-assisted drug delivery is a common and effective method that has been used to increase the efficiency of transdermal drug delivery by selectively destroying the chromophores of interest using light waves.
Specifications for using LADD
Lasers
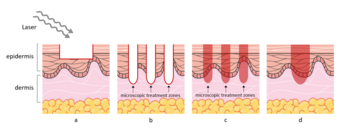
There are different types of lasers used in LADD, and they can be categorized into four main groups: (1) fully ablative lasers, (2) ablative fractional lasers (AFL), (3) non-ablative fractional lasers (NAFL), and (4) non-ablative dermal remodeling lasers.[6] Common fully ablative lasers, including carbon dioxide (CO₂, wavelength peak 10,600 nm) and erbium-doped yttrium aluminum garnet (Er:YAG, wavelength peak 2940 nm), target water as their chromophore where all water-containing tissues within the epidermis are ablated.[7] With its high wavelength peak, CO₂ laser has a high absorption rate of water and adipose tissues; whereas the wavelength of Er:YAG allows for the precise ablation of water and minimizes heat generation.[8] The mechanism of AFL is similar to fully ablative lasers but when used fractionally, they create multiple vertical columns on the skin surface, which are also called microscopic treatment zones (MTZ) and allow for a more quantitatively controllable usage in LADD.[8] NAFL are also fractionated lasers that produce MTZ, but as they are non-ablative, there is no ablation of the epidermis and instead, they use light energy to damage the dermis layer.[9] Non-ablative dermal remodeling lasers include all types of lasers with a chromophore that is different from water as used in the previous groups. Common lasers within this group are neodymium-doped YAG laser (Nd: YAG, wavelength peak 1064 nm and 1320 nm), pulsed dye laser (wavelength ranges from 585 to 600 nm), and intense pulsed laser (IPL, wavelength ranges from 500 to 1200 nm).[9] In general, with its high efficiency and rapid recovery time, AFL is the more common modality used for LADD, especially in the dermatology field.[10]
Drugs
Lipophilic substances have shown to have a greater ability to cross the epidermis, thus, the efficiency of LADD is more remarkable when using hydrophilic substances.[6][11] Liquid and gel formulations of drugs also are proven to cross the channels created from the fractional lasers more easily as compared to oily formulations such as creams or ointments.[6] Common drugs used in LAPP include but are not limited to 5-aminolaevulinic (5-ALA), 5-aminolevulinate (MAL), methotrexate (MTX), imiquimod, 5-fluorouracil (5-FU), timolol, triamcinolone acetonide (TAC), bimatoprost, tretinoin, pimecrolimus, poly-L-lactic acid (PLLA), analgesics, minoxidil (MXD), diphencyprone (DPCP), vitamin C, small interfering RNA (siRNA), vaccine, and antibodies.[12]
Patients
The efficiency of LADD with the selected laser settings is dependent upon the different characteristics associated with individual patients. The dermatological condition, the properties of the skin, and the surface area are taken into consideration to determine the eligibility of the patients for certain lasers and provide optimal treatments for each patient. For example, hydrated skin has a higher affinity for absorption of oily substances; skin atrophy that is associated with solar elastosis is more likely to produce pathological scarring under high laser intensity; hair areas have a higher absorption rate; older patients are more prone to adverse effects such as atrophy, erosion, ulceration, and will require longer recovery time.[6] Not all patients are candidates for LADD as this method is intensified as compared to conventional topical treatment.[13]
Pre-clinical application
A vast majority of pre-clinical work on LADD focuses on AFL based on its translational characteristics in clinical settings. These studies utilized mostly either porcine or murine skin as their disease model.
Porcine skin
Within the dermatology field, porcine skin has been used as a disease model for testing the efficacy of LADD in vivo. Haedersdal et al. pre-treated porcine skin with CO2-AFL before the application of MAL photodynamic therapy (PDT), creating single MTZs that increased porphyrin fluorescence uniformly up to 1.5 mm from the ablated channels.[14] This demonstrated that for MAL, pre-treatment of AFL with MTZs spacing at 3-mm intervals, covering less than 1% surface of the area, was useful for the entire lesion.[7] Similarly, Bachhav et al. showed that the increased numbers of MTZs from Er:YAG laser did not affect the absorption of lidocaine into either the epidermis or dermis, and thus, higher fluences of laser were not proportionally correlated to the absorption rate.[15] AFL pre-treatment of porcine skin also has also shown to enhance the delivery of MAL at deeper layers of the skin,[15] increase surface fluorescence from MAL as compared to non-AFL pre-treated skin,[16] and induce higher fluorescence of 5-ALA as compared to MAL for deeper structure.[17]
Murine skin
Besides porcine skin, murine skin has also been used for testing the efficacy of LADD. A study performed on murine skin has shown that the penetration of 5-FU through skin increased 36 to 133-fold after pre-treatment with fully ablative Q-switched ruby, CO2, or Er:YAG lasers.[18] Likewise, delivery of imiquimod in both murine and porcine skin increased up to 65-fold and 127-fold, after one and four passes of low-fluence fractional Er:YAG laser, respectively.[19] As a result, with LADD, a dose of 0.4% imiquimod was equivalent to a topically applied dose of 5% imiquimod,[19] which implied that a lower dosage of drug could be used with similar clinical outcomes. Besides topical drugs, Chen et al. showed that the treatment of fractional CO2 on murine skin increased 8- to 15-fold the delivery of ovalbumin vaccine, along with an enhanced production of ovalbumin specific antibodies at 2 weeks.[20]
Clinical application
LADD has been implemented in clinical practice to support the absorption of topical agents into the skin, representative drugs include 5-ALA, MAL, 5-FU, corticosteroid, vitamin, and lidocaine.
Photodynamic Therapy
LADD has been used in adjunction to photodynamic therapy (PDT) as a pre-treatment, which has shown to enhance the absorption of these drugs into the skin. 5-ALA and MAL are common photosensitizers that are used in PDT to treat different skin diseases such as actinic keratoses (AK), Bowen’s disease, and superficial cell carcinoma.[12]
5-ALA
Lim et al. (2014) utilized nonablative fractional laser Er:YAG to pre-treat twelve treatment areas on the back of 10 healthy males, followed by the incubation of 5-ALA.[21] The results showed that pre-treated areas had higher level of porphyrin fluorescence as compared to non-pretreated areas, which indicated that LADD enhanced 5-ALA skin penetration. In another study, Jang et al. (2013) pre-treated 29 AK patients with an ablative CO2 fractional laser, followed by 5-ALA-PDT treatment with varying incubation times.[22] The pre-treatment of laser showed improvement of clinical outcomes even with the short incubation time, with 70.6% of the AK lesions had a complete clinical response to PDT.
MAL
In a randomized study, Choi et al. used both conventional MAL-PDT and a combination of AFL (Er:YAG) and MAL-PDT to treat 93 AK patients.[23] The group treated with a combination of LADD and PDT showed higher clinical response rate of 91.7% as compared to conventional MAL-PDT group with clinical response rate of 65.6% after three months, and results were persistent after a twelve-month follow-up. In another randomized study with 21 patients with Bowen’s disease, the clearance rate of the lesions after 3 months was higher with pre-treatment of one session of ablative fractional Er:YAG followed by MAL-PDT (93.8%) as compared to two sessions of conventional MAL-PDT (73.1%), and with lower recurrence rates (6.7% versus 31.6%).[24]
5-FU
5-FU is a common drug which is used to treat cancer and certain skin diseases, such as AK and certain types of nonmelanoma skin cancers. In a case study of 28 patients, including 16 superficial basal cell carcinomas and 14 squamous cell carcinomas in situ, pre-treatment of a single pass of CO2-AFL followed by a single application of 5-FU showed histological clearance of 100% squamous cell carcinomas in situ and 71% of the superficial basal cell carcinomas.[25] In a case report study, a patient with multiple Bowen’s disease lesions was selected for a half-side study, one was treated with Er:YAG laser followed by a topical treatment of 5-FU and the other was treated with only 5-FU cream.[26] The legions treated with LADD showed accelerated clinical and histologic response as compared to conventional 5-FU, with no recurrences of lesions after 9 months. In another study, Wenande et al. (2021) showed that CO2-AFL enhanced the efficiency of cisplatin and 5-FU treatment for 20 patients with basal cell carcinoma, with 94.7% patients showed clinical clearance.[27]
Corticosteroid
Triamcinolone acetonide (TAC) is a common corticosteroid used as a therapeutic strategy for hypertrophic scars and keloids.[28] In a case study, Waibel et al. used CO2-AFL to assist topical TAC delivery to treat 15 patients with hypertrophic and restrictive cutaneous scars. The results showed that significant improvement of the scars was observed after 6 months, with the most impacts on texture.[29] Similarly, in a pilot study, either CO2-AFL or radiofrequency was used in adjunction with ultrasound-assisted TAC to treat alopecia areata, a disease associated with hair loss.[30] All patients showed complete response after the treatment and specifically, the use of LADD with CO2-AFL showed complete response of patients after a single session as compared to a required of three and six sessions for radiofrequency.
Vitamin
Vitamin C and E are important substances that show antioxidant effects against UV radiation. Transdermal delivery after topical application of these vitamins has been facilitated with LADD. Lee et al. (2003) showed that the application of either nonablative fractional Er:YAG or CO2 lasers improved the transdermal penetration of vitamin C significantly.[31] In a split face comparison study regarding UV-induced skin aging, Trelles et al. treated 14 patients with conventional CO2-AFL on one side and CO2-AFL along with the application of vitamin C and E on the other side.[32] As a result, the combination of LADD and vitamins demonstrated a 79% reduction in fine lines as compared to a 69% reduction for AFL-treated side without the delivery of vitamins.
Lidocaine
Local anesthesia is used widely for dermatological surgeries via topical products or injections. As topical agents have a long incubation time for drug penetration and injections are associated with pain, LADD has been applied to the field for advancing the efficacy of anesthesia. Lidocaine is a local anesthetic cream used to prevent and treat pain. Increase dermal absorption and transdermal bioavailability of lidocaine were seen when using in conjunction with LADD, specifically AFL. Yun et al. demonstrated that 5% lidocaine cream applied after Er:YAG-AFL for a full resurfacing procedure showed significant lower pain score after the first pass of resurfacing, but there were only half of the patients were able to tolerate the second pass.[33] Nevertheless, this indicated that LADD showed an enhancement of lidocaine penetration through the stratum corneum. Similarly, in a double-blind randomized controlled trial with 320 healthy volunteers, the patients were either pre-treated with Er:YAG-AFL followed by 4% lidocaine or treated with topical 4% lidocaine alone before cannulation.[34] The results showed a 62% reduction in pain with the use of LADD as compared to conventional topical lidocaine.
Safety and Adverse Events
When applying LADD in clinical settings, safety is an imperative factor that needs to be considered. As mentioned above, there are different lasers with distinct properties that could be implemented for patients. However, radiation of any type will damage human tissue to some extent. Some potential adverse events that are laser-induced include erythema, edema, scabbing, blistering, and pigmentary changes, especially at higher intensity and densities.[35][36][37] Regarding intralesional therapies for scarring, telangiectasia, hypopigmentation, and skin atrophy have been observed in multiple studies as side effects of LADD.[35][38][39][40][41] In LADD application for management of pigmentary condition, there are some pigment-related adverse effects such as worsening of melasma,[42][43] and hyperpigmentation in vitiligo.[44][45] While LADD improves the dermal infiltration of different medications, the skin also has heightened local reaction when exposed to the substances and external environment.[46] In several studies about AFL-assisted delivery of MAL-photodynamic therapy, there are intensified local cutaneous responses, including burning sensation, pain, edema, pruritus, purpura, and transient pigmentary changes.[47][48][49][50][51][52] Furthermore, exposing the underlying dermis and vasculature to the outside environment also increases the risk of infection.[26] AFL-assisted delivery of 5-FU, steroids, and MAL have shown elevated bacterial infections.[53][54][28][55][56] In general, most LADD safety concerns are related to local reactions similar to that of laser therapy, and are generally well-tolerated with some exceptions.[57]
Future Direction
Current studies support the use of LADD in adjunction with topical products in treating dermatological diseases, but these studies are limited in sample size and lack of long-term follow-up outcomes. Larger randomized controlled trials with a wide variety of topical drugs are required to validate the efficacy and side effects of LADD before this technique could be employed as a standard of treatment. Besides its application in drug delivery, the prospect of using LADD to improve the transdermal delivery of vaccines, promote wound healing, correct genetic sequence, and as a complement to inflammatory dermatoses and cosmetic indications is being investigated[58]
References
- ↑ 1.0 1.1 1.2 Lopez-Ojeda, Wilfredo; Pandey, Amarendra; Alhajj, Mandy; Oakley, Amanda M. (2023), "Anatomy, Skin (Integument)", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 28723009, http://www.ncbi.nlm.nih.gov/books/NBK441980/, retrieved 2023-04-22
- ↑ 2.0 2.1 2.2 2.3 2.4 Alkilani, Ahlam; McCrudden, Maelíosa T.; Donnelly, Ryan (2015-10-22). "Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum" (in en). Pharmaceutics 7 (4): 438–470. doi:10.3390/pharmaceutics7040438. ISSN 1999-4923. PMID 26506371.
- ↑ Arora, Anubhav; Prausnitz, Mark R.; Mitragotri, Samir (December 2008). "Micro-scale devices for transdermal drug delivery" (in en). International Journal of Pharmaceutics 364 (2): 227–236. doi:10.1016/j.ijpharm.2008.08.032. PMID 18805472.
- ↑ Donnelly, Ryan F.; Singh, Thakur Raghu Raj; Garland, Martin J.; Migalska, Katarzyna; Majithiya, Rita; McCrudden, Cian M.; Kole, Prashant Laxman; Mahmood, Tuan Mazlelaa Tuan et al. (2012-12-05). "Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery" (in en). Advanced Functional Materials 22 (23): 4879–4890. doi:10.1002/adfm.201200864. PMID 23606824.
- ↑ Jeong, Woo Yeup; Kwon, Mina; Choi, Hye Eun; Kim, Ki Su (December 2021). "Recent advances in transdermal drug delivery systems: a review" (in en). Biomaterials Research 25 (1): 24. doi:10.1186/s40824-021-00226-6. ISSN 2055-7124. PMID 34321111.
- ↑ 6.0 6.1 6.2 6.3 Alegre-Sánchez, A.; Jiménez-Gómez, N.; Boixeda, P. (December 2018). "Laser-Assisted Drug Delivery" (in en). Actas Dermo-Sifiliográficas (English Edition) 109 (10): 858–867. doi:10.1016/j.adengl.2018.10.012. PMID 30266385. https://linkinghub.elsevier.com/retrieve/pii/S1578219018303664.
- ↑ 7.0 7.1 Ali, Faisal R.; Al-Niaimi, Firas (February 2016). "Laser-assisted drug delivery in dermatology: from animal models to clinical practice" (in en). Lasers in Medical Science 31 (2): 373–381. doi:10.1007/s10103-015-1853-z. ISSN 0268-8921. PMID 26694489. http://link.springer.com/10.1007/s10103-015-1853-z.
- ↑ 8.0 8.1 Goo, Boncheol Leo (2015-12-30). "Laser Assisted Drug and Cosmeceutical Delivery System of the Skin" (in en). Medical Lasers 4 (2): 51–59. doi:10.25289/ML.2015.4.2.51. ISSN 2287-8300. http://www.jkslms.or.kr/journal/view.html?doi=10.25289/ML.2015.4.2.51.
- ↑ 9.0 9.1 Pozner, J (September 2002). "Continuing Medical Education Article—Facial Aesthetic Surgery: Nonablative Laser Resurfacing: State of the Art 2002" (in en). Aesthetic Surgery Journal 22 (5): 427–434. doi:10.1067/maj.2002.128619. PMID 19331996. https://academic.oup.com/asj/article-lookup/doi/10.1067/maj.2002.128619.
- ↑ Alexiades-Armenakas, Macrene R.; Dover, Jeffrey S.; Arndt, Kenneth A. (May 2008). "The spectrum of laser skin resurfacing: Nonablative, fractional, and ablative laser resurfacing". Journal of the American Academy of Dermatology 58 (5): 719–737. doi:10.1016/j.jaad.2008.01.003. ISSN 0190-9622. PMID 18423256. http://dx.doi.org/10.1016/j.jaad.2008.01.003.
- ↑ Franz, Thomas J. (November 1983). "Kinetics of Cutaneous Drug Penetration" (in en). International Journal of Dermatology 22 (9): 499–505. doi:10.1111/j.1365-4362.1983.tb02187.x. ISSN 0011-9059. PMID 6358066. https://onlinelibrary.wiley.com/doi/10.1111/j.1365-4362.1983.tb02187.x.
- ↑ 12.0 12.1 Zaleski-Larsen, Lisa Ann; Fabi, Sabrina G. (August 2016). "Laser-Assisted Drug Delivery" (in en). Dermatologic Surgery 42 (8): 919–931. doi:10.1097/DSS.0000000000000556. ISSN 1076-0512. PMID 27191783. https://journals.lww.com/00042728-201608000-00001.
- ↑ Wenande, Emily; Anderson, R. Rox; Haedersdal, Merete (January 2020). "Fundamentals of fractional laser-assisted drug delivery: An in-depth guide to experimental methodology and data interpretation" (in en). Advanced Drug Delivery Reviews 153: 169–184. doi:10.1016/j.addr.2019.10.003. PMID 31628965. https://linkinghub.elsevier.com/retrieve/pii/S0169409X19301851.
- ↑ Haedersdal, Merete; Sakamoto, Fernanda H.; Farinelli, William A.; Doukas, Apostolos G.; Tam, Josh; Anderson, R. Rox (February 2010). "Fractional CO 2 laser-assisted drug delivery: FRACTIONAL CO 2 LASER-ASSISTED DRUG DELIVERY" (in en). Lasers in Surgery and Medicine 42 (2): 113–122. doi:10.1002/lsm.20860. PMID 20166154. https://onlinelibrary.wiley.com/doi/10.1002/lsm.20860.
- ↑ 15.0 15.1 Haedersdal, M.; Katsnelson, J.; Sakamoto, F.H.; Farinelli, W.A.; Doukas, A.G.; Tam, J.; Anderson, R.R. (September 2011). "Enhanced uptake and photoactivation of topical methyl aminolevulinate after fractional CO2 laser pretreatment" (in en). Lasers in Surgery and Medicine 43 (8): 804–813. doi:10.1002/lsm.21096. PMID 21956628. https://onlinelibrary.wiley.com/doi/10.1002/lsm.21096.
- ↑ Haak, Christina S.; Farinelli, William A.; Tam, Joshua; Doukas, Apostolos G.; Anderson, R. Rox; Haedersdal, Merete (December 2012). "Fractional laser-assisted delivery of methyl aminolevulinate: Impact of laser channel depth and incubation time" (in en). Lasers in Surgery and Medicine 44 (10): 787–795. doi:10.1002/lsm.22102. PMID 23212624. https://onlinelibrary.wiley.com/doi/10.1002/lsm.22102.
- ↑ Haedersdal, Merete; Sakamoto, Fernanda H.; Farinelli, William A.; Doukas, Apostolos G.; Tam, Joshua; Anderson, R. Rox (August 2014). "Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL): PRETREATMENT WITH AFXL CHANGES OF ALA AND MAL" (in en). Lasers in Surgery and Medicine 46 (6): 462–469. doi:10.1002/lsm.22259. PMID 24842112. https://onlinelibrary.wiley.com/doi/10.1002/lsm.22259.
- ↑ Lee, Woan‐Ruoh; Shen, Shing‐Chuan; Wang, Kuo‐Hsien; Hu, Chung‐Hong; Fang, Jia‐You (July 2002). "The Effect of Laser Treatment on Skin to Enhance and Control Transdermal Delivery of 5‐Fluorouracil" (in en). Journal of Pharmaceutical Sciences 91 (7): 1613–1626. doi:10.1002/jps.10142. PMID 12115823. https://linkinghub.elsevier.com/retrieve/pii/S0022354916310462.
- ↑ 19.0 19.1 Lee, Woan-Ruoh; Shen, Shing-Chuan; Al-Suwayeh, Saleh A.; Yang, Hung-Hsu; Yuan, Cheng-Yin; Fang, Jia-You (August 2011). "Laser-assisted topical drug delivery by using a low-fluence fractional laser: Imiquimod and macromolecules" (in en). Journal of Controlled Release 153 (3): 240–248. doi:10.1016/j.jconrel.2011.03.015. PMID 21435360. https://linkinghub.elsevier.com/retrieve/pii/S0168365911001519.
- ↑ Chen, Xinyuan; Shah, Dilip; Kositratna, Garuna; Manstein, Dieter; Anderson, Richard R.; Wu, Mei X. (April 2012). "Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology" (in en). Journal of Controlled Release 159 (1): 43–51. doi:10.1016/j.jconrel.2012.01.002. PMID 22261281.
- ↑ Lim, H.K.; Jeong, K.H.; Kim, N.I.; Shin, M.K. (June 2014). "Nonablative fractional laser as a tool to facilitate skin penetration of 5-aminolaevulinic acid with minimal skin disruption: a preliminary study" (in en). British Journal of Dermatology 170 (6): 1336–1340. doi:10.1111/bjd.12817. PMID 24386881. https://academic.oup.com/bjd/article/170/6/1336/6614715.
- ↑ Jang, Yong Hyun; Lee, Dong Jun; Shin, Jaeyoung; Kang, Hee Young; Lee, Eun-So; Kim, You Chan (2013). "Photodynamic Therapy with Ablative Carbon Dioxide Fractional Laser in Treatment of Actinic Keratosis" (in en). Annals of Dermatology 25 (4): 417–422. doi:10.5021/ad.2013.25.4.417. ISSN 1013-9087. PMID 24371387.
- ↑ Choi, S.H.; Kim, K.H.; Song, K.H. (August 2015). "Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial" (in en). Journal of the European Academy of Dermatology and Venereology 29 (8): 1598–1605. doi:10.1111/jdv.12953. PMID 25640401. https://onlinelibrary.wiley.com/doi/10.1111/jdv.12953.
- ↑ Ko, D.Y.; Kim, K.H.; Song, K.H. (January 2014). "A randomized trial comparing methyl aminolaevulinate photodynamic therapy with and without Er:YAG ablative fractional laser treatment in Asian patients with lower extremity Bowen disease: results from a 12-month follow-up" (in en). British Journal of Dermatology 170 (1): 165–172. doi:10.1111/bjd.12627. PMID 24102369. https://academic.oup.com/bjd/article/170/1/165/6614856.
- ↑ Nguyen, Bichchau T.; Gan, Stephanie D.; Konnikov, Nellie; Liang, Christine A. (March 2015). "Treatment of superficial basal cell carcinoma and squamous cell carcinoma in situ on the trunk and extremities with ablative fractional laser-assisted delivery of topical fluorouracil" (in en). Journal of the American Academy of Dermatology 72 (3): 558–560. doi:10.1016/j.jaad.2014.11.033. PMID 25687314. https://linkinghub.elsevier.com/retrieve/pii/S0190962214022191.
- ↑ 26.0 26.1 Wang, Kuo-Hsien; Fang, Jia-You; Hu, Chung-Hong; Lee, Woan-Ruoh (March 2004). "Erbium:YAG Laser Pretreatment Accelerates the Response of Bowen's Disease Treated by Topical 5-Fluorouracil" (in en). Dermatologic Surgery 30 (3): 441–445. doi:10.1111/j.1524-4725.2004.30122.x. ISSN 1076-0512. PMID 15008880. https://journals.lww.com/10.1111/j.1524-4725.2004.30122.x.
- ↑ Wenande, Emily; Hendel, Kristoffer; Mogensen, Mette; Bagger, Charlotte; Mårtensson, Nina L.; Persson, Daniel P.; Lerche, Catharina M.; Husted, Søren et al. (January 2021). "Efficacy and Safety of Laser‐Assisted Combination Chemotherapy: An Explorative Imaging‐Guided Treatment With 5‐Fluorouracil and Cisplatin for Basal Cell Carcinoma" (in en). Lasers in Surgery and Medicine 53 (1): 119–128. doi:10.1002/lsm.23323. ISSN 0196-8092. PMID 32960987. https://onlinelibrary.wiley.com/doi/10.1002/lsm.23323.
- ↑ 28.0 28.1 Braun, Stephan Alexander; Schrumpf, Holger; Buhren, Bettina Alexandra; Homey, Bernhard; Gerber, Peter Arne (May 2016). "Laser-assisted drug delivery: mode of action and use in daily clinical practice: Laser-assisted drug delivery" (in en). JDDG: Journal der Deutschen Dermatologischen Gesellschaft 14 (5): 480–488. doi:10.1111/ddg.12963. PMID 27119468. https://onlinelibrary.wiley.com/doi/10.1111/ddg.12963.
- ↑ Waibel, Jill S.; Wulkan, Adam J.; Shumaker, Peter R. (March 2013). "Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery: TREATMENT OF HYPERTROPHIC SCARS" (in en). Lasers in Surgery and Medicine 45 (3): 135–140. doi:10.1002/lsm.22120. PMID 23460557. https://onlinelibrary.wiley.com/doi/10.1002/lsm.22120.
- ↑ Issa, Maria Claudia Almeida; Pires, Marianna; Silveira, Priscilla; Xavier de Brito, Esther; Sasajima, Cristiane (2015-01-02). "Transepidermal drug delivery: A new treatment option for areata alopecia?" (in en). Journal of Cosmetic and Laser Therapy 17 (1): 37–40. doi:10.3109/14764172.2014.967778. ISSN 1476-4172. PMID 25260052. http://www.tandfonline.com/doi/full/10.3109/14764172.2014.967778.
- ↑ Lee, Woan-Ruoh; Shen, Shing-Chuan; Wang, Kuo-Hsien; Hu, Chung-Hong; Fang, Jia-You (November 2003). "Lasers and Microdermabrasion Enhance and Control Topical Delivery of Vitamin C" (in en). Journal of Investigative Dermatology 121 (5): 1118–1125. doi:10.1046/j.1523-1747.2003.12537.x. PMID 14708614. https://linkinghub.elsevier.com/retrieve/pii/S0022202X1530525X.
- ↑ Trelles, M. A.; Leclère, F. M.; Martínez-Carpio, P. A. (October 2013). "Fractional Carbon Dioxide Laser and Acoustic-Pressure Ultrasound for Transepidermal Delivery of Cosmeceuticals: A Novel Method of Facial Rejuvenation" (in en). Aesthetic Plastic Surgery 37 (5): 965–972. doi:10.1007/s00266-013-0176-3. ISSN 0364-216X. PMID 23812612. http://link.springer.com/10.1007/s00266-013-0176-3.
- ↑ Yun, Patricia Lee; Tachihara, Rieko; Anderson, R.Rox (October 2002). "Efficacy of erbium:yttrium-aluminum-garnet laser-assisted delivery of topical anesthetic" (in en). Journal of the American Academy of Dermatology 47 (4): 542–547. doi:10.1067/mjd.2002.124819. PMID 12271298. https://linkinghub.elsevier.com/retrieve/pii/S0190962202000956.
- ↑ Baron, Elma D. (2003-10-01). "Laser-Assisted Penetration of Topical Anesthetic in Adults" (in en). Archives of Dermatology 139 (10): 1288–1290. doi:10.1001/archderm.139.10.1288. ISSN 0003-987X. PMID 14568832. http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.139.10.1288.
- ↑ 35.0 35.1 Park, Ji Hye; Chun, Ji Young; Lee, Jong Hee (April 2017). "Laser-assisted topical corticosteroid delivery for the treatment of keloids" (in en). Lasers in Medical Science 32 (3): 601–608. doi:10.1007/s10103-017-2154-5. ISSN 0268-8921. PMID 28124198. http://link.springer.com/10.1007/s10103-017-2154-5.
- ↑ Seo, Hyun-Min; Choi, Ju-Yeon; Min, Jung; Kim, Won-Serk (2016-04-02). "Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas" (in en). Journal of Cosmetic and Laser Therapy 18 (3): 149–153. doi:10.3109/14764172.2015.1052517. ISSN 1476-4172. PMID 26073121. http://www.tandfonline.com/doi/full/10.3109/14764172.2015.1052517.
- ↑ Mohamed, Hager A.; Mohammed, Ghada F.; Gomaa, Amal H. A.; Eyada, Moustafa M. K. (2015-07-04). "Carbon dioxide laser plus topical 5-fluorouracil: a new combination therapeutic modality for acral vitiligo" (in en). Journal of Cosmetic and Laser Therapy 17 (4): 216–223. doi:10.3109/14764172.2014.1003241. ISSN 1476-4172. PMID 25549816. http://www.tandfonline.com/doi/full/10.3109/14764172.2014.1003241.
- ↑ Sabry, Hanan Hassan; Ibrahim, Eman Ahmed; Hamed, Ahmed Mohamed (November 2020). "Assessment of laser‐assisted delivery vs intralesional injection of botulinum toxin A in treatment of hypertrophic scars and keloids" (in en). Dermatologic Therapy 33 (6): e13980. doi:10.1111/dth.13980. ISSN 1396-0296. PMID 32638463. https://onlinelibrary.wiley.com/doi/10.1111/dth.13980.
- ↑ Wang, Jue; Wu, Jiang; Xu, Minghuo; Gao, Quanwen; Chen, Baoguo; Wang, Fang; Song, Huifeng (November 2020). "Combination therapy of refractory keloid with ultrapulse fractional carbon dioxide ( CO 2 ) laser and topical triamcinolone in Asians‐ long‐term prevention of keloid recurrence" (in en). Dermatologic Therapy 33 (6): e14359. doi:10.1111/dth.14359. ISSN 1396-0296. PMID 33002270. https://onlinelibrary.wiley.com/doi/10.1111/dth.14359.
- ↑ Abd El‐Dayem, Dina H.; Nada, Hesham A.; Hanafy, Noha S.; Elsaie, Mohamed L. (January 2021). "Laser‐assisted topical steroid application versus steroid injection for treating keloids: A split side study" (in en). Journal of Cosmetic Dermatology 20 (1): 138–142. doi:10.1111/jocd.13521. ISSN 1473-2130. PMID 32485049. https://onlinelibrary.wiley.com/doi/10.1111/jocd.13521.
- ↑ Manuskiatti, W; Kaewkes, A; Yan, C; Ng, J; Glahn, J; Wanitphakdeedecha, R (2021). "Hypertrophic Scar Outcomes in Fractional Laser Monotherapy Versus Fractional Laser-Assisted Topical Corticosteroid Delivery: A Randomized Clinical Trial" (in en). Acta Dermato Venereologica 101 (3): adv00416. doi:10.2340/00015555-3781. ISSN 1651-2057. PMID 33686446. PMC 9366502. https://medicaljournalssweden.se/actadv/article/view/967.
- ↑ Wanitphakdeedecha, Rungsima; Sy-Alvarado, Francesca; Patthamalai, Poramin; Techapichetvanich, Thanya; Eimpunth, Sasima; Manuskiatti, Woraphong (December 2020). "The efficacy in treatment of facial melasma with thulium 1927-nm fractional laser-assisted topical tranexamic acid delivery: a split-face, double-blind, randomized controlled pilot study" (in en). Lasers in Medical Science 35 (9): 2015–2021. doi:10.1007/s10103-020-03045-8. ISSN 0268-8921. PMID 32506227. https://link.springer.com/10.1007/s10103-020-03045-8.
- ↑ Botsali, Aysenur; Esme, Pelin; Erbil, Hakan; Caliskan, Ercan (2022-03-26). "Comparison of fractional erbium:YAG laser-assisted tranexamic acid delivery alone and in combination with oral tranexamic acid in melasma" (in en). Lasers in Medical Science 37 (7): 2823–2830. doi:10.1007/s10103-022-03547-7. ISSN 1435-604X. PMID 35347552.
- ↑ Huang, Chuchu; Li, Peiyao; Wang, Ben; Deng, Yuxuan; Li, Ji; Mao, Mengping; Jian, Dan (September 2020). "Multi‐Factors Associated With Efficacy and Adverse Events of Fractional Erbium:YAG Laser‐Assisted Delivery of Topical Betamethasone for Stable Vitiligo: A Retrospective Analysis" (in en). Lasers in Surgery and Medicine 52 (7): 590–596. doi:10.1002/lsm.23198. ISSN 0196-8092. PMID 31820470. https://onlinelibrary.wiley.com/doi/10.1002/lsm.23198.
- ↑ Doghaim, Noha Nabil; El‐Tatawy, Rania Ahmed; Ismail, Mayada A.; Ali, Dareen Abdelaziz Mohammed; El Attar, Yasmina Ahmed (January 2020). "Study the effect of erbium:YAG laser plus topical 5‐flurouracil in stable vitiligo resistant to NB‐UVB phototherapy" (in en). Journal of Cosmetic Dermatology 19 (1): 122–130. doi:10.1111/jocd.13134. ISSN 1473-2130. PMID 31571367. https://onlinelibrary.wiley.com/doi/10.1111/jocd.13134.
- ↑ Ibrahim, Omer; Wenande, Emily; Hogan, Sara; Arndt, Kenneth A.; Haedersdal, Merete; Dover, Jeffrey S. (January 2018). "Challenges to laser-assisted drug delivery: Applying theory to clinical practice: CHALLENGES TO LASER-ASSISTED DRUG DELIVERY" (in en). Lasers in Surgery and Medicine 50 (1): 20–27. doi:10.1002/lsm.22769. PMID 29154501. https://onlinelibrary.wiley.com/doi/10.1002/lsm.22769.
- ↑ Helsing, P.; Togsverd-Bo, K.; Veierød, M.B.; Mørk, G.; Haedersdal, M. (November 2013). "Intensified fractional CO 2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands" (in en). British Journal of Dermatology 169 (5): 1087–1092. doi:10.1111/bjd.12507. PMID 23855503. https://academic.oup.com/bjd/article/169/5/1087/6615299.
- ↑ Togsverd-Bo, K.; Lei, U.; Erlendsson, A.M.; Taudorf, E.H.; Philipsen, P.A.; Wulf, H.C.; Skov, L.; Haedersdal, M. (February 2015). "Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients - a randomized controlled trial" (in en). British Journal of Dermatology 172 (2): 467–474. doi:10.1111/bjd.13222. PMID 24975199. https://academic.oup.com/bjd/article/172/2/467/6615762.
- ↑ Lippert, Jan; Šmucler, Roman; Vlk, Marek (August 2013). "Fractional Carbon Dioxide Laser Improves Nodular Basal Cell Carcinoma Treatment with Photodynamic Therapy with Methyl 5-Aminolevulinate" (in en). Dermatologic Surgery 39 (8): 1202–1208. doi:10.1111/dsu.12242. ISSN 1076-0512. PMID 23725586. https://journals.lww.com/00042728-201308000-00010.
- ↑ Song, Hyo Sang; Jung, Soo-Eun; Jang, Yong Hyun; Kang, Hee Young; Lee, Eun-So; Kim, You Chan (November 2015). "Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis" (in en). Photodermatology, Photoimmunology & Photomedicine 31 (6): 296–301. doi:10.1111/phpp.12184. PMID 26012779. https://onlinelibrary.wiley.com/doi/10.1111/phpp.12184.
- ↑ Choi, S.H.; Kim, K.H.; Song, K.H. (May 2016). "Er:YAG ablative fractional laser-primed photodynamic therapy with methyl aminolevulinate as an alternative treatment option for patients with thin nodular basal cell carcinoma: 12-month follow-up results of a randomized, prospective, comparative trial" (in en). Journal of the European Academy of Dermatology and Venereology 30 (5): 783–788. doi:10.1111/jdv.13453. PMID 26551044. https://onlinelibrary.wiley.com/doi/10.1111/jdv.13453.
- ↑ Choi, Seung-Hwan; Kim, Ki-Ho; Song, Ki-Hoon (2017-03-01). "Effect of Methyl Aminolevulinate Photodynamic Therapy With and Without Ablative Fractional Laser Treatment in Patients With Microinvasive Squamous Cell Carcinoma: A Randomized Clinical Trial" (in en). JAMA Dermatology 153 (3): 289–295. doi:10.1001/jamadermatol.2016.4463. ISSN 2168-6068. PMID 28199463. http://archderm.jamanetwork.com/article.aspx?doi=10.1001/jamadermatol.2016.4463.
- ↑ Mohamed, Hager A.; Mohammed, Ghada F.; Gomaa, Amal H. A.; Eyada, Moustafa M. K. (2015-07-04). "Carbon dioxide laser plus topical 5-fluorouracil: a new combination therapeutic modality for acral vitiligo" (in en). Journal of Cosmetic and Laser Therapy 17 (4): 216–223. doi:10.3109/14764172.2014.1003241. ISSN 1476-4172. PMID 25549816. http://www.tandfonline.com/doi/full/10.3109/14764172.2014.1003241.
- ↑ Togsverd-Bo, K.; Haak, C.S.; Thaysen-Petersen, D.; Wulf, H.C.; Anderson, R.R.; Haedesdal, M. (June 2012). "Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial: Intensified PDT of AK with fractional CO2 laser" (in en). British Journal of Dermatology 166 (6): 1262–1269. doi:10.1111/j.1365-2133.2012.10893.x. PMID 22348388. https://academic.oup.com/bjd/article/166/6/1262/6613835.
- ↑ Vachiramon, Vasanop; Chaiyabutr, Chayada; Rattanaumpawan, Pinyo; Kanokrungsee, Silada (February 2016). "Effects of a preceding fractional carbon dioxide laser on the outcome of combined local narrowband ultraviolet B and topical steroids in patients with vitiligo in difficult-to-treat areas: FRACTIONAL CO 2 LASER AND NARROWBAND UVB FOR VITILIGO" (in en). Lasers in Surgery and Medicine 48 (2): 197–202. doi:10.1002/lsm.22389. PMID 26175036. https://onlinelibrary.wiley.com/doi/10.1002/lsm.22389.
- ↑ Torezan, Luís; Chaves, Yuri; Niwa, Ane; Sanches, José A.; Festa-Neto, Cyro; Szeimies, Rolf-Markus (August 2013). "A Pilot Split-Face Study Comparing Conventional Methyl Aminolevulinate-Photodynamic Therapy (PDT) With Microneedling-Assisted PDT on Actinically Damaged Skin" (in en). Dermatologic Surgery 39 (8): 1197–1201. doi:10.1111/dsu.12233. ISSN 1076-0512. PMID 23638986. https://journals.lww.com/00042728-201308000-00009.
- ↑ Ng, William Hao Syuen; Smith, Saxon D. (2022-12-07). "Laser-Assisted Drug Delivery: A Systematic Review of Safety and Adverse Events" (in en). Pharmaceutics 14 (12): 2738. doi:10.3390/pharmaceutics14122738. ISSN 1999-4923. PMID 36559233.
- ↑ Brauer, Jeremy; Krakowski, Andrew; Bloom, Bradley; Nguyen, Tuyet; Geronemus, Roy (December 2014). "Convergence of anatomy, technology, and therapeutics: a review of laser-assisted drug delivery". Seminars in Cutaneous Medicine and Surgery 33 (4): 176–181. doi:10.12788/j.sder.0119. PMID 25830250. http://scmsjournal.com/article/buy_now/?id=42.
 |