Medicine:Numerical Stroop effect

The numerical Stroop effect, a concept rooted in cognitive psychology, refers to the interference that occurs when individuals are asked to compare numerical values or physical sizes of digits presented together. The effect arises when there is a mismatch—or incongruity—between the numerical value and the physical size of the digits. For example, comparing a physically larger "3" and a smaller "5" can result in slower reaction times, as the brain encounters conflicting information between size and value. Conversely, response times are faster when the size and value align, such as a large "5" and a small "3".
This phenomenon is conceptually linked to the traditional Stroop effect, which involves interference between word meaning and font color. However, unlike the standard Stroop effect—where the interference is asymmetrical—the numerical Stroop effect exhibits a more balanced pattern. Both irrelevant size and irrelevant numerical values can interfere with task performance, indicating that numerical and physical size processing occur in parallel. The numerical Stroop effect also highlights the automatic nature of number processing, which can persist even when participants are explicitly instructed to ignore one dimension (e.g., size).
Studies using functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) have demonstrated that brain regions such as the intraparietal sulcus are crucial for processing this effect, with heightened activation observed during incongruent trials. The effect is further modulated by individual cognitive development, as children may respond differently depending on their familiarity with numerical symbols. Research into the numerical Stroop effect has implications for understanding the neural basis of numerical cognition and contributes to broader studies on cognitive interference and brain function.
Original experiments
Besner and Coltheart (1979) asked participants to compare values and ignore the sizes of the digits (i.e., the numerical task). They reported that the irrelevant sizes slowed down responding when sizes were incongruent with the values of the digits.[1] Henik and Tzelgov (1982) examined not only the numerical task but also the physical task. The numerical Stroop effect was found in both tasks. Moreover, when the two dimensions were congruent, responding was facilitated (relative to neutral trials) and when the two dimensions were incongruent, responding was slower (relative to neutral trials).[2]
Experimental findings
The original Stroop effect is asymmetrical - color responses are slowed down by irrelevant words but word reading is commonly not affected by irrelevant colors.[3][4] Unlike the Stroop effect, the numerical Stroop effect is symmetrical – irrelevant sizes affect the comparisons of values and irrelevant values affect comparisons of sizes. The latter gave rise to the suggestion that values are processed automatically because this occurs even when responding to values is much slower than responding to sizes.[2] Moreover, processing values depends on familiarity with the numerical symbolic system. Accordingly, young children may show the size effect in numerical comparisons but not the effect of values in physical size comparisons.[5][6]
Neuroanatomy
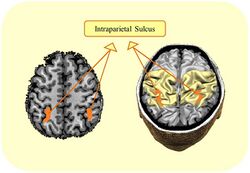
Functional magnetic resonance imaging (fMRI) studies have pinpointed the brain regions that are involved in the numerical Stroop effect.[7][8][9] In these studies the most consistent finding was the involvement of the parietal cortex, with increased activation for incongruent in comparison to congruent trials. When a neutral condition was included, it was observed that the bilateral parietal lobes were the only regions that were involved in both facilitation and interference.[10] Electroencephalography (EEG) studies[11][12][13] have indicated that the amplitude or the latency of the P300 wave is modulated as a function of the congruity effect. This means that when looking at amplitude, the difference between the amplitude of the congruent and incongruent condition is observed 300 ms after the presentation of the digits. In addition, behavioral, physiological, and computational studies support the view, although not unanimously,[11] that the conflict between congruent and incongruent conditions is observed up to the response level,[12][14][15][16][17] and is dependent on the developmental stage of the participant.[13]
The above-mentioned studies allow inferring the neural correlate of the numerical Stroop effect. However, they do not allow concluding whether parietal lobe function is critical for this effect. Brain stimulation studies that use techniques such as transcranial magnetic stimulation or transcranial direct current stimulation allow modulating parietal lobe function and inferring its role. These studies have suggested that the right parietal lobe in particular is necessary for the numerical Stroop effect,[18][19] albeit stimulation of the right parietal lobe might affect other connected brain regions. Moreover, work with acquired acalculia[20] suggested involvement of the left parietal lobe in the numerical Stroop effect. This effect is commonly reduced in cases of brain damage to the left intraparietal sulcus.
References
- ↑ Besner, Derek; Coltheart, Max (1979). "Ideographic and alphabetic processing in skilled reading of English". Neuropsychologia 17 (5): 467–472. doi:10.1016/0028-3932(79)90053-8. PMID 514483.
- ↑ 2.0 2.1 Henik, Avishai; Tzelgov, Joseph (July 1982). "Is three greater than five: The relation between physical and semantic size in comparison tasks". Memory & Cognition 10 (4): 389–395. doi:10.3758/BF03202431. PMID 7132716.
- ↑ MacLeod, C. M. (1991). "Half a century of research on the Stroop effect: An integrative review". Psychological Bulletin 109 (2): 163–203. doi:10.1037/0033-2909.109.2.163. PMID 2034749.
- ↑ Stroop, J. R. (1935). "Studies of interference in serial verbal reactions". Journal of Experimental Psychology 18 (6): 643–662. doi:10.1037/h0054651.
- ↑ Girelli, Luisa; Lucangeli, Daniela; Butterworth, Brian (June 2000). "The Development of Automaticity in Accessing Number Magnitude". Journal of Experimental Child Psychology 76 (2): 104–122. doi:10.1006/jecp.2000.2564. PMID 10788305.
- ↑ Rubinsten, Orly; Henik, Avishai; Berger, Andrea; Shahar-Shalev, Sharon (2002). "The development of internal representations of magnitude and their association with Arabic numerals". Journal of Experimental Child Psychology 81 (1): 74–92. doi:10.1006/jecp.2001.2645. PMID 11741375.
- ↑ Pinel, P; Piazza, M; Le Bihan, D; Dehaene, S (2004). "Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments". Neuron 41 (6): 983–993. doi:10.1016/S0896-6273(04)00107-2. PMID 15046729.
- ↑ Kaufmann, L; Koppelstaetter, F; Delazer, M; Siedentopf, C; Rhomberg, P; Golaszewski, S; Felber, S; Ischebeck, A (2005). "Neural correlates of distance and congruity effects in a numerical Stroop task: An event-related fMRI study". NeuroImage 25 (3): 888–898. doi:10.1016/j.neuroimage.2004.12.041. PMID 15808989.
- ↑ Cohen Kadosh, R; Cohen Kadosh, K; Henik, A (2008). "When brightness counts: The neuronal correlate of numerical-luminance interference". Cerebral Cortex 18 (2): 337–343. doi:10.1093/cercor/bhm058. PMID 17556772.
- ↑ Cohen Kadosh, R; Cohen Kadosh, K; Henik, A; Linden, D.E.J (2008). "Processing conflicting information: Facilitation, interference, and functional connectivity". Neuropsychologia 46 (12): 2872–2879. doi:10.1016/j.neuropsychologia.2008.05.025. PMID 18632120.
- ↑ 11.0 11.1 Gebuis, T; Leon Kenemans, J; de Haan, E.H.F; van der Smagt, M.J (2010). "Conflict processing of symbolic and non-symbolic numerosity". Neuropsychologia 48 (2): 394–401. doi:10.1016/j.neuropsychologia.2009.09.027. PMID 19804788.
- ↑ 12.0 12.1 Cohen Kadosh, R; Cohen Kadosh, K; Linden, D.E.J; Gevers, W; Berger, A; Henik, A (2007). "The brain locus of interaction between number and size: A combined functional magnetic resonance imaging and event-related potential study". Journal of Cognitive Neuroscience 19 (6): 957–970. doi:10.1162/jocn.2007.19.6.957. PMID 17536966.
- ↑ 13.0 13.1 Szucs, D; Soltesz, F; Jarmi, E; Csepe, V (2007). "The speed of magnitude processing and executive functions in controlled and automatic number comparison in children: An electro-encephalography study". Behavioral and Brain Functions 3: 23. doi:10.1186/1744-9081-3-23. PMID 17470279.
- ↑ Szucs, D; Soltesz, F; White, S (2009). "Motor conflict in Stroop tasks: Direct evidence from single-trial electro-myography and electro-encephalography". NeuroImage 47 (4): 1960–1973. doi:10.1016/j.neuroimage.2009.05.048. PMID 19481157. https://eprints.qut.edu.au/32343/1/c32343.pdf.
- ↑ Cohen Kadosh, R; Gevers, W; Notebaert, W (2011). "Sequential analysis of the numerical Stroop effect reveals response suppression". Journal of Experimental Psychology: Learning, Memory, and Cognition 37 (5): 1243–1249. doi:10.1037/a0023550. PMID 21500951.
- ↑ Santens, S; Verguts, T (2011). "The size congruity effect: is bigger always more?". Cognition 118 (1): 94–110. doi:10.1016/j.cognition.2010.10.014. PMID 21074146.
- ↑ Szucs, D; Soltesz, F (2007). "Event-related potentials dissociate facilitation and interference effects in the numerical Stroop paradigm". Neuropsychologia 45 (14): 3190–3202. doi:10.1016/j.neuropsychologia.2007.06.013. PMID 17675108.
- ↑ Cohen Kadosh, R; Cohen Kadosh, K; Schuhmann, T; Kaas, A; Goebel, R; Henik, A; Sack, A.T (2007). "Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing". Current Biology 17 (8): 689–693. doi:10.1016/j.cub.2007.02.056. PMID 17379521. Bibcode: 2007CBio...17..689C. https://cris.maastrichtuniversity.nl/en/publications/ce2b3646-cef9-43e6-b88f-0289852e2a2e.
- ↑ Cohen Kadosh, R; Soskic, S; Iuculano, T; Kanai, R; Walsh, V (2010). "Modulating neuronal activity produces specific and long lasting changes in numerical competence". Current Biology 20 (22): 2016–2020. doi:10.1016/j.cub.2010.10.007. PMID 21055945. Bibcode: 2010CBio...20.2016C.
- ↑ Ashkenazi, S; Henik, A; Ifergane, G; Shelef, I (2008). "Basic numerical processing in left intraparietal sulcus (IPS) acalculia". Cortex 44 (4): 439–448. doi:10.1016/j.cortex.2007.08.008. PMID 18387576.
 |
