Physics:Transcranial magnetic stimulation
| Transcranial magnetic stimulation | |
|---|---|
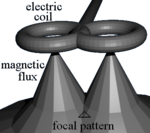
 Transcranial magnetic stimulation (schematic diagram) | |
| Specialty | Psychiatry, neurology |
| MeSH | D050781 |
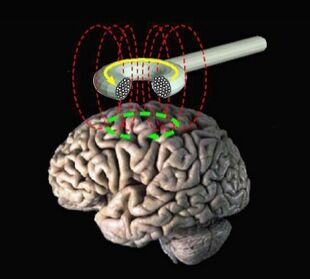
Transcranial magnetic stimulation (TMS) is a noninvasive form of brain stimulation in which a changing magnetic field is used to induce an electric current at a specific area of the brain through electromagnetic induction. An electric pulse generator, or stimulator, is connected to a magnetic coil connected to the scalp. The stimulator generates a changing electric current within the coil which creates a varying magnetic field, inducing a current within a region in the brain itself.[1]:3[2]
TMS has shown diagnostic and therapeutic potential in the central nervous system with a wide variety of disease states in neurology and mental health, with research still evolving.[3][4][5][6][7][8][9][10][11]
Adverse effects of TMS appear rare and include fainting and seizure.[12] Other potential issues include discomfort, pain, hypomania, cognitive change, hearing loss, and inadvertent current induction in implanted devices such as pacemakers or defibrillators.[12]
Medical uses

TMS does not require surgery or electrode implantation.
Its use can be diagnostic and/or therapeutic. Effects vary based on frequency and intensity of the magnetic pulses as well as the length of treatment, which dictates the total number of pulses given.[14] TMS treatments are approved by the FDA in the US and by NICE in the UK for the treatment of depression and are predominantly provided by private clinics. TMS stimulates cortical tissue without the pain sensations produced in transcranial electrical stimulation.[15][better source needed]
Diagnosis
TMS can be used clinically to measure activity and function of specific brain circuits in humans, most commonly with single or paired magnetic pulses.[3] The most widely accepted use is in measuring the connection between the primary motor cortex of the central nervous system and the peripheral nervous system to evaluate damage related to past or progressive neurologic insult.[3][16][17][18]
Treatment
Repetitive high frequency TMS (rTMS) has shown diagnostic and therapeutic potential with the central nervous system in a variety of disease states, particularly in the fields of neurology and mental health.[3][4][5][7][8][9][10]
Adverse effects
Although TMS is generally regarded as safe, risks are increased for therapeutic rTMS compared to single or paired diagnostic TMS.[19] Adverse effects generally increase with higher frequency stimulation.[12]
The greatest immediate risk from TMS is fainting, though this is uncommon. Seizures have been reported, but are rare.[12][20][21] Other adverse effects include short term discomfort, pain, brief episodes of hypomania, cognitive change, hearing loss, impaired working memory, and the induction of electrical currents in implanted devices such as cardiac pacemakers.[12]
Procedure
During the procedure, a magnetic coil is positioned at the head of the person receiving the treatment using anatomical landmarks on the skull, in particular the inion and nasion.[13] The coil is then connected to a pulse generator, or stimulator, that delivers electric current to the coil.[2]
Physics
TMS uses electromagnetic induction to generate an electric current across the scalp and skull.[22][23] A plastic-enclosed coil of wire is held next to the skull and when activated, produces a varying magnetic field oriented orthogonally to the plane of the coil. The changing magnetic field then induces an electric current in the brain that activates nearby nerve cells in a manner similar to a current applied superficially at the cortical surface.[24]
The magnetic field is about the same strength as magnetic resonance imaging (MRI), and the pulse generally reaches no more than 5 centimeters into the brain unless using a modified coil and technique for deeper stimulation.[23]
Transcranial magnetic stimulation is achieved by quickly discharging current from a large capacitor into a coil to produce pulsed magnetic fields between 2 and 3 teslas in strength.[25] Directing the magnetic field pulse at a targeted area in the brain causes a localized electrical current which can then either depolarize or hyperpolarize neurons at that site. The induced electric field inside the brain tissue causes a change in transmembrane potentials resulting in depolarization or hyperpolarization of neurons, causing them to be more or less excitable, respectively.[25]
Deep TMS can reach up to 6 cm into the brain to stimulate deeper layers of the motor cortex, such as that which controls leg motion. The path of this current can be difficult to model because the brain is irregularly shaped with variable internal density and water content, leading to a nonuniform magnetic field strength and conduction throughout its tissues.[26]
Frequency and duration
The effects of TMS can be divided based on frequency, duration and intensity (amplitude) of stimulation:[27]
- Single or paired pulse TMS causes neurons in the neocortex under the site of stimulation to depolarize and discharge an action potential. If used in the primary motor cortex, it produces muscle activity referred to as a motor evoked potential (MEP) which can be recorded on electromyography. If used on the occipital cortex, 'phosphenes' (flashes of light) might be perceived by the subject. In most other areas of the cortex, there is no conscious effect, but behaviour may be altered (e.g., slower reaction time on a cognitive task), or changes in brain activity may be detected using diagnostic equipment.[28]
- Repetitive TMS produces longer-lasting effects which persist past the period of stimulation. rTMS can increase or decrease the excitability of the corticospinal tract depending on the intensity of stimulation, coil orientation, and frequency. Low frequency rTMS with a stimulus frequency less than 1 Hz is believed to inhibit cortical firing while a stimulus frequency greater than 1 Hz, or high frequency, is believed to provoke it.[29] Though its mechanism is not clear, it has been suggested as being due to a change in synaptic efficacy related to long-term potentiation (LTP) and long-term depression like plasticity (LTD-like plasticity).[30][31]
Coil types
Most devices use a coil shaped like a figure-eight to deliver a shallow magnetic field that affects more superficial neurons in the brain.[9] Differences in magnetic coil design are considered when comparing results, with important elements including the type of material, geometry and specific characteristics of the associated magnetic pulse.
The core material may be either a magnetically inert substrate ('air core'), or a solid, ferromagnetically active material ('solid core'). Solid cores result in more efficient transfer of electrical energy to a magnetic field and reduce energy loss to heat, and so can be operated with the higher volume of therapy protocols without interruption due to overheating. Varying the geometric shape of the coil itself can cause variations in focality, shape, and depth of penetration. Differences in coil material and its power supply also affect magnetic pulse width and duration.[32]
A number of different types of coils exist, each of which produce different magnetic fields. The round coil is the original used in TMS. Later, the figure-eight (butterfly) coil was developed to provide a more focal pattern of activation in the brain, and the four-leaf coil for focal stimulation of peripheral nerves. The double-cone coil conforms more to the shape of the head.[33] The Hesed (H-core), circular crown and double cone coils allow more widespread activation and a deeper magnetic penetration. They are supposed to impact deeper areas in the motor cortex and cerebellum controlling the legs and pelvic floor, for example, though the increased depth comes at the cost of a less focused magnetic pulse.[12]
History
Luigi Galvani (1737–1798) undertook research on the effects of electricity on the body in the late-eighteenth century and laid the foundations for the field of electrophysiology.[34] In the 1830s Michael Faraday (1791–1867) discovered that an electrical current had a corresponding magnetic field, and that changing one could induce its counterpart.[35]
Work to directly stimulate the human brain with electricity started in the late 1800s, and by the 1930s the Italian physicians Cerletti and Bini had developed electroconvulsive therapy (ECT).[34] ECT became widely used to treat mental illness, and ultimately overused, as it began to be seen as a panacea. This led to a backlash in the 1970s.[34]
In 1980 Merton and Morton successfully used transcranial electrical stimulation (TES) to stimulate the motor cortex. However, this process was very uncomfortable, and subsequently Anthony T. Barker began to search for an alternative to TES.[36] He began exploring the use of magnetic fields to alter electrical signaling within the brain, and the first stable TMS devices were developed in 1985.[34][35] They were originally intended as diagnostic and research devices, with evaluation of their therapeutic potential being a later development.[34][35] The United States' FDA first approved TMS devices in October 2008.[34]
Research
TMS has shown potential therapeutic effect on neurologic conditions such as mild to moderate Alzheimer's disease,[4] amyotrophic lateral sclerosis,[4][37] persistent vegetative states,[4] epilepsy,[4][38] stroke related disability,[4][12][17][18][39][40] tinnitus,[4][41] multiple sclerosis,[4] schizophrenia,[4][10] and traumatic brain injury.[42]
With Parkinson's disease, early results suggest that low frequency stimulation may have an effect on medication associated dyskinesia, and that high frequency stimulation improves motor function.[43][44] The most effective treatment protocols appear to involve high frequency stimulation of the motor cortex, particularly on the dominant side,[45] but with more variable results for treatment of the dorsolateral prefrontal cortex.[46] It is less effective than electroconvulsive therapy for motor symptoms, though both appear to have utility.[47][48][49] Cerebellar stimulation has also shown potential for the treatment of levodopa associated dyskinesia.[50]
In psychiatry, it has shown potential with anxiety disorders, including panic disorder[51] and obsessive–compulsive disorder (OCD).[4] The most promising areas to target for OCD appear to be the orbitofrontal cortex and the supplementary motor area.[52] Older protocols that targeted the prefrontal dorsal cortex were less successful.[53] It has also been studied with autism,[54] substance abuse,[4] addiction,[4][55][56] and post-traumatic stress disorder (PTSD).[4] For treatment-resistant major depressive disorder, high-frequency (HF) rTMS of the left dorsolateral prefrontal cortex (DLPFC) appears effective and low-frequency (LF) rTMS of the right DLPFC has probable efficacy.[4][5][7][8][9] Research on the efficacy of rTMS in non-treatment-resistant depression is limited.[57]
TMS can also be used to map functional connectivity between the cerebellum and other areas of the brain.[58]
A study at the Wahrendorff Clinic in Germany in 2021[59] reported that 84% of participants in the study have experienced positive effects after using the treatment.
Under the supervision of Professor Marc Ziegenbein a psychiatry and psychotherapy specialist, the study of 77 subjects with mild to moderate Alzheimer’s disease received frequent transcranial magnetic stimulation applications and observed over a period of time.
Improvements were mainly found in the areas of orientation in the environment, concentration, general well-being and satisfaction.
Study blinding
Mimicking the physical discomfort of TMS with placebo to discern its true effect is a challenging issue in research.[4][12][60][61] It is difficult to establish a convincing placebo for TMS during controlled trials in conscious individuals due to the neck pain, headache and twitching in the scalp or upper face associated with the intervention.[4][12] In addition, placebo manipulations can affect brain sugar metabolism and MEPs, which may confound results.[62] This problem is exacerbated when using subjective measures of improvement.[12] Placebo responses in trials of rTMS in major depression are negatively associated with refractoriness to treatment.[63]
A 2011 review found that most studies did not report unblinding. In the minority that did, participants in real and sham rTMS groups were not significantly different in their ability to correctly guess their therapy, though there was a trend for participants in the real group to more often guess correctly.[64]
Animal model limitations
TMS research in animal studies is limited due to its early US Food and Drug Administration approval for treatment-resistant depression, limiting development of animal specific magnetic coils.[65]
Treatments for the general public
Regulatory approvals
Neurosurgery planning
Nexstim obtained United States Federal Food, Drug, and Cosmetic Act§Section 510(k) clearance for the assessment of the primary motor cortex for pre-procedural planning in December 2009[66] and for neurosurgical planning in June 2011.[67]
Depression
The National Institutes of Health estimates depression medications work for 60 percent to 70 percent of people who take them.[68][69] It is approved as a Class II medical device under the "de novo pathway".[70][71] In addition, the World Health Organization reports that the number of people living with depression has increased nearly 20 percent since 2005.[72] In a 2012 study, TMS was found to improve depression significantly in 58 percent of patients and provide complete remission of symptoms in 37 percent of patients.[73] In 2002, Cochrane Library reviewed randomized controlled trials using TMS to treat depression. The review did not find a difference between rTMS and sham TMS, except for a period 2 weeks after treatment.[74] In 2018, Cochrane Library stated a plan to contact authors about updating the review of rTMS for depression.[75]
Obsessive–compulsive disorder (OCD)
In August 2018, the US Food and Drug Administration (US FDA) authorized the use of TMS developed by the Israeli company Brainsway in the treatment of obsessive–compulsive disorder (OCD).[76]
In 2020, US FDA authorized the use of TMS developed by the U.S. company MagVenture Inc. in the treatment of OCD.[77]
In 2023, US FDA authorized the use of TMS developed by the U.S. company Neuronetics Inc. in the treatment of OCD.[78]
Other neurological areas
In the European Economic Area, various versions of Deep TMS H-coils have CE marking for Alzheimer's disease,[79] autism,[79] bipolar disorder,[80] epilepsy,[81] chronic pain,[80] major depressive disorder,[80] Parkinson's disease,[45][82] post-traumatic stress disorder (PTSD),[80][83] schizophrenia (negative symptoms)[80] and to aid smoking cessation.[79] One review found tentative benefit for cognitive enhancement in healthy people.[84]
Coverage by health services and insurers
United Kingdom
The United Kingdom's National Institute for Health and Care Excellence (NICE) issues guidance to the National Health Service (NHS) in England, Wales, Scotland and Northern Ireland (UK). NICE guidance does not cover whether or not the NHS should fund a procedure. Local NHS bodies (primary care trusts and hospital trusts) make decisions about funding after considering the clinical effectiveness of the procedure and whether the procedure represents value for money for the NHS.[85]
NICE evaluated TMS for severe depression (IPG 242) in 2007, and subsequently considered TMS for reassessment in January 2011 but did not change its evaluation.[86] The Institute found that TMS is safe, but there is insufficient evidence for its efficacy.[86]
In January 2014, NICE reported the results of an evaluation of TMS for treating and preventing migraine (IPG 477). NICE found that short-term TMS is safe but there is insufficient evidence to evaluate safety for long-term and frequent uses. It found that evidence on the efficacy of TMS for the treatment of migraine is limited in quantity, that evidence for the prevention of migraine is limited in both quality and quantity.[87]
Subsequently, in 2015, NICE approved the use of TMS for the treatment of depression in the UK and IPG542 replaced IPG242.[88] NICE said "The evidence on repetitive transcranial magnetic stimulation for depression shows no major safety concerns. The evidence on its efficacy in the short-term is adequate, although the clinical response is variable. Repetitive transcranial magnetic stimulation for depression may be used with normal arrangements for clinical governance and audit."
United States: commercial health insurance
In 2013, several commercial health insurance plans in the United States, including Anthem, Health Net, and Blue Cross Blue Shield of Nebraska and of Rhode Island, covered TMS for the treatment of depression for the first time.[89][90][91][92] In contrast, UnitedHealthcare issued a medical policy for TMS in 2013 that stated there is insufficient evidence that the procedure is beneficial for health outcomes in patients with depression. UnitedHealthcare noted that methodological concerns raised about the scientific evidence studying TMS for depression include small sample size, lack of a validated sham comparison in randomized controlled studies, and variable uses of outcome measures.[93] Other commercial insurance plans whose 2013 medical coverage policies stated that the role of TMS in the treatment of depression and other disorders had not been clearly established or remained investigational included Aetna, Cigna and Regence.[94][95][96]
United States: Medicare
Policies for Medicare coverage vary among local jurisdictions within the Medicare system,[97] and Medicare coverage for TMS has varied among jurisdictions and with time. For example:
- In early 2012 in New England, Medicare covered TMS for the first time in the United States.[98][99][100][101] However, that jurisdiction later decided to end coverage after October, 2013.[102]
- In August 2012, the jurisdiction covering Arkansas, Louisiana, Mississippi, Colorado, Texas, Oklahoma, and New Mexico determined that there was insufficient evidence to cover the treatment,[103] but the same jurisdiction subsequently determined that Medicare would cover TMS for the treatment of depression after December 2013.[104]
- Subsequently,[when?] some other Medicare jurisdictions added Medicare coverage for depression.
See also
- Cortical stimulation mapping
- Cranial electrotherapy stimulation
- Electrical brain stimulation
- Electroconvulsive therapy
- Low field magnetic stimulation
- My Beautiful Broken Brain
- Non-invasive cerebellar stimulation
- Transcranial alternating current stimulation
- Transcranial direct-current stimulation
- Transcranial random noise stimulation
- Vagus nerve stimulation
References
- ↑ NICE. January 2014 Transcranial magnetic stimulation for treating and preventing migraine
- ↑ Jump up to: 2.0 2.1 Michael Craig Miller for Harvard Health Publications. July 26, 2012 Magnetic stimulation: a new approach to treating depression?
- ↑ Jump up to: 3.0 3.1 3.2 3.3 "A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee". Clinical Neurophysiology 123 (5): 858–882. May 2012. doi:10.1016/j.clinph.2012.01.010. PMID 22349304.
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 "Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS)". Clinical Neurophysiology 125 (11): 2150–2206. November 2014. doi:10.1016/j.clinph.2014.05.021. PMID 25034472. https://hal.archives-ouvertes.fr/hal-03183867/file/S1388245719312799.pdf.
- ↑ Jump up to: 5.0 5.1 5.2 George, Mark S.; Post, Robert M. (April 2011). "Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation for Acute Treatment of Medication-Resistant Depression". American Journal of Psychiatry 168 (4): 356–364. doi:10.1176/appi.ajp.2010.10060864. PMID 21474597.
- ↑ Gaynes, Bradley N.; Lux, Linda J.; Lloyd, Stacey W.; Hansen, Richard A.; Gartlehner, Gerald; Keener, Patricia; Brode, Shannon; Evans, Tammeka Swinson et al. (2011). Nonpharmacologic Interventions for Treatment-Resistant Depression in Adults. AHRQ Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality. https://www.ncbi.nlm.nih.gov/books/NBK65315/.
- ↑ Jump up to: 7.0 7.1 7.2 Berlim, Marcelo T; Van den Eynde, Frederique; Jeff Daskalakis, Z (19 November 2012). "Clinically Meaningful Efficacy and Acceptability of Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) for Treating Primary Major Depression: A Meta-Analysis of Randomized, Double-Blind and Sham-Controlled Trials". Neuropsychopharmacology 38 (4): 543–551. doi:10.1038/npp.2012.237. PMID 23249815.
- ↑ Jump up to: 8.0 8.1 8.2 Perera, Tarique; George, Mark; Grammer, Geoffrey; Janicak, Philip; Pascual-Leone, Alvaro; Wirecki, Theodore (April 27, 2015). TMS Therapy For Major Depressive Disorder: Evidence Review and Treatment Recommendations for Clinical Practice (Report). http://tmscenterofcolorado.com/wp-content/uploads/2015/09/Clinical-TMS-Society-WhitePaper_2015.pdf.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 Bersani, F.S.; Minichino, A.; Enticott, P.G.; Mazzarini, L.; Khan, N.; Antonacci, G.; Raccah, R.N.; Salviati, M. et al. (January 2013). "Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: A comprehensive review". European Psychiatry 28 (1): 30–39. doi:10.1016/j.eurpsy.2012.02.006. PMID 22559998.
- ↑ Jump up to: 10.0 10.1 10.2 "Transcranial magnetic stimulation (TMS) for schizophrenia". The Cochrane Database of Systematic Reviews 2015 (8): CD006081. August 2015. doi:10.1002/14651858.CD006081.pub2. PMID 26289586. PMC 9395125. http://dspace.stir.ac.uk/bitstream/1893/22520/1/Dougall_et_al-2015-The_Cochrane_Library.pdf.
- ↑ "Pupil response may shed light on who responds best to transcranial magnetic stimulation for depression" (in en). https://www.uclahealth.org/news/pupil-response-may-shed-light-who-responds-best-transcranial.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 Rossi (January 2021). "Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines.". Clinical Neurophysiology 132 (1): 269–306. doi:10.1016/j.clinph.2020.10.003. PMID 33243615.
- ↑ Jump up to: 13.0 13.1 "Assessment of standard coil positioning in transcranial magnetic stimulation in depression". Psychiatry Research 186 (2–3): 232–238. April 2011. doi:10.1016/j.psychres.2010.06.012. PMID 20692709.
- ↑ Klomjai, Wanalee; Katz, Rose; Lackmy-Vallée, Alexandra (2015-09-01). "Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS)" (in en). Annals of Physical and Rehabilitation Medicine 58 (4): 208–213. doi:10.1016/j.rehab.2015.05.005. ISSN 1877-0657. PMID 26319963.
- ↑ Moliadze, V., Zhao, Y., Eysel, U. and Funke, K., 2003. "Effect of transcranial magnetic stimulation on single‐unit activity in the cat primary visual cortex". The Journal of Physiology, 553(2), pp. 665–679.
- ↑ "Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential". Neurology 68 (7): 484–488. February 2007. doi:10.1212/01.wnl.0000250268.13789.b2. PMID 17296913.
- ↑ Jump up to: 17.0 17.1 "Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke". Neurorehabilitation and Neural Repair 24 (2): 125–135. February 2010. doi:10.1177/1545968309345270. PMID 19767591.
- ↑ Jump up to: 18.0 18.1 "Noninvasive brain stimulation and motor recovery after stroke". Restorative Neurology and Neuroscience 28 (4): 531–544. 2010. doi:10.3233/RNN-2010-0552. PMID 20714076.
- ↑ "Recognizing the risks of brain stimulation". Science 346 (6215): 1307. December 2014. doi:10.1126/science.346.6215.1307-a. PMID 25504707.
- ↑ "Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion". Neuropsychiatric Disease and Treatment 11: 2975–2987. 2015. doi:10.2147/NDT.S91126. PMID 26664122.
- ↑ "7. rTMS-Associated Adverse Events". Repetitive Transcranial Magnetic Stimulation for Depressive Disorders. Berlin Heidelberg: Springer-Verlag. 2013. pp. 81–90. doi:10.1007/978-3-642-36467-9. ISBN 978-3-642-36466-2. https://books.google.com/books?id=2VFEAAAAQBAJ.
- ↑ "The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis". Systematic Reviews 6 (1): 48. March 2017. doi:10.1186/s13643-017-0440-8. PMID 28264713.
- ↑ Jump up to: 23.0 23.1 "Brain Stimulation Therapies". https://www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml.
- ↑ Handbook of psychophysiology (3rd ed.). New York: Cambridge Univ. Press. 2007. p. 121. ISBN 978-0-521-84471-0.
- ↑ Jump up to: 25.0 25.1 V. Walsh and A. Pascual-Leone, "Transcranial Magnetic Stimulation: A Neurochronometrics of Mind." Cambridge, Massachusetts: MIT Press, 2003.
- ↑ See:
- "Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil". Clinical Neurophysiology 116 (4): 775–779. April 2005. doi:10.1016/j.clinph.2004.11.008. PMID 15792886.
- "Consensus: New methodologies for brain stimulation". Brain Stimulation 2 (1): 2–13. January 2009. doi:10.1016/j.brs.2008.09.007. PMID 20633398.
- ↑ "Parameterization of transcranial magnetic stimulation". Journal of Neurophysiology 107 (5): 1257–1259. March 2012. doi:10.1152/jn.00716.2011. PMID 22072509.
- ↑ Handbook of Transcranial Magnetic Stimulation. London: Edward Arnold. 2002. ISBN 978-0-340-72009-7.
- ↑ "Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS". Biology of Mood & Anxiety Disorders 2 (1): 14. August 2012. doi:10.1186/2045-5380-2-14. PMID 22901565.
- ↑ "A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition". Clinical Neurophysiology 117 (12): 2584–2596. December 2006. doi:10.1016/j.clinph.2006.06.712. PMID 16890483.
- ↑ Baur D, Galevska D, Hussain S, Cohen LG, Ziemann U, Zrenner C. Induction of LTD-like corticospinal plasticity by low-frequency rTMS depends on pre-stimulus phase of sensorimotor μ-rhythm. Brain Stimul. 2020 Nov-Dec;13(6):1580-1587. doi: 10.1016/j.brs.2020.09.005. Epub 2020 Sep 17. PMID: 32949780; PMCID: PMC7710977.
- ↑ "TMS Stimulator Design". Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press. 2008. pp. 13–23, 25–32. ISBN 978-0-19-856892-6.
- ↑ "In vitro evaluation of a 4-leaf coil design for magnetic stimulation of peripheral nerve". Electroencephalography and Clinical Neurophysiology 93 (1): 68–74. February 1994. doi:10.1016/0168-5597(94)90093-0. PMID 7511524.
- ↑ Jump up to: 34.0 34.1 34.2 34.3 34.4 34.5 "Transcranial magnetic stimulation: a historical evaluation and future prognosis of therapeutically relevant ethical concerns". Journal of Medical Ethics 37 (3): 137–143. March 2011. doi:10.1136/jme.2010.039966. PMID 21106996.
- ↑ Jump up to: 35.0 35.1 35.2 "History, Studies and Specific Uses of Repetitive Transcranial Magnetic Stimulation (rTMS) in Treating Epilepsy". Iranian Journal of Child Neurology 10 (1): 1–8. 2016. PMID 27057180.
- ↑ "Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS)". Annals of Physical and Rehabilitation Medicine 58 (4): 208–213. September 2015. doi:10.1016/j.rehab.2015.05.005. PMID 26319963.
- ↑ "Repetitive transcranial magnetic stimulation for the treatment of amyotrophic lateral sclerosis or motor neuron disease". The Cochrane Database of Systematic Reviews 2013 (5): CD008554. May 2013. doi:10.1002/14651858.CD008554.pub3. PMID 23728676.
- ↑ "Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review". Epilepsy & Behavior 57 (Pt A): 167–176. April 2016. doi:10.1016/j.yebeh.2016.01.015. PMID 26970993.
- ↑ "Research with transcranial magnetic stimulation in the treatment of aphasia". Current Neurology and Neuroscience Reports 9 (6): 451–458. November 2009. doi:10.1007/s11910-009-0067-9. PMID 19818232.
- ↑ "Repetitive transcranial magnetic stimulation of motor cortex after stroke: a focused review". American Journal of Physical Medicine & Rehabilitation 91 (3): 254–270. March 2012. doi:10.1097/PHM.0b013e318228bf0c. PMID 22042336.
- ↑ "Transcranial magnetic stimulation: a new diagnostic and therapeutic tool for tinnitus patients". The International Tinnitus Journal 14 (2): 112–118. 2008. PMID 19205161.
- ↑ "Neurostimulation for traumatic brain injury". Journal of Neurosurgery 121 (5): 1219–1231. November 2014. doi:10.3171/2014.7.JNS131826. PMID 25170668.
- ↑ "Therapeutic applications of repetitive transcranial magnetic stimulation in clinical neurorehabilitation". Functional Neurology 23 (3): 113–122. 2008. PMID 19152730.
- ↑ Liu, Xuan; Li, Lei; Liu, Ye (2023-09-29). "Comparative motor effectiveness of non-invasive brain stimulation techniques in patients with Parkinson's disease: A network meta-analysis". Medicine 102 (39): e34960. doi:10.1097/MD.0000000000034960. ISSN 1536-5964. PMID 37773851. PMC 10545289. https://pubmed.ncbi.nlm.nih.gov/37773851/.
- ↑ Jump up to: 45.0 45.1 "Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: a systematic review and meta-analysis". JAMA Neurology 72 (4): 432–440. April 2015. doi:10.1001/jamaneurol.2014.4380. PMID 25686212.
- ↑ "Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson's disease: A Meta-analysis". Brain and Behavior 8 (11): e01132. November 2018. doi:10.1002/brb3.1132. PMID 30264518.
- ↑ "Non-invasive brain stimulation for Parkinson's disease: a systematic review and meta-analysis of the literature". Journal of Neurology, Neurosurgery, and Psychiatry 76 (12): 1614–1623. December 2005. doi:10.1136/jnnp.2005.069849. PMID 16291882.
- ↑ "Treatment of Parkinson's disease by cortical stimulation". Expert Review of Neurotherapeutics 9 (12): 1755–1771. December 2009. doi:10.1586/ern.09.132. PMID 19951135.
- ↑ "Basic mechanisms of rTMS: Implications in Parkinson's disease". International Archives of Medicine 1 (1): 2. April 2008. doi:10.1186/1755-7682-1-2. PMID 18471317.
- ↑ "Effects of cerebellar neuromodulation in movement disorders: A systematic review". Brain Stimulation 11 (2): 249–260. 2018. doi:10.1016/j.brs.2017.11.015. PMID 29191439.
- ↑ "Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults". The Cochrane Database of Systematic Reviews 9 (9): CD009083. September 2014. doi:10.1002/14651858.CD009083.pub2. PMID 25230088.
- ↑ "Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials". Journal of Psychiatric Research 47 (8): 999–1006. August 2013. doi:10.1016/j.jpsychires.2013.03.022. PMID 23615189.
- ↑ "Transcranial cortical stimulation in the treatment of obsessive-compulsive disorders: efficacy studies". Current Psychiatry Reports 17 (5): 36. May 2015. doi:10.1007/s11920-015-0571-3. PMID 25825002.
- ↑ "Transcranial magnetic stimulation in autism spectrum disorder: Challenges, promise, and roadmap for future research". Autism Research 9 (2): 184–203. February 2016. doi:10.1002/aur.1567. PMID 26536383.
- ↑ "Non-invasive stimulation therapies for the treatment of refractory pain". Discovery Medicine 14 (74): 21–31. July 2012. PMID 22846200. http://www.discoverymedicine.com/Julien-Nizard/2012/07/24/non-invasive-stimulation-therapies-for-the-treatment-of-refractory-pain/.
- ↑ Ponciano-Rodríguez, Guadalupe; Chávez-Castillo, Carlos A.; Ríos-Ponce, Alma E.; Villafuerte, Gabriel (2021). "High Frequency and Low Intensity Transcranial Magnetic Stimulation for Smoking Cessation". Journal of Addiction 2021: 9988618. doi:10.1155/2021/9988618. ISSN 2090-7834. PMID 34589245.
- ↑ Kiebs, Maximilian; Hurlemann, René; Mutz, Julian (August 2019). "Repetitive transcranial magnetic stimulation in non-treatment-resistant depression" (in en). British Journal of Psychiatry 215 (2): 445–446. doi:10.1192/bjp.2019.75. ISSN 0007-1250. PMID 31014413.
- ↑ "Targeting the Cerebellum by Noninvasive Neurostimulation: a Review". Cerebellum 16 (3): 695–741. June 2017. doi:10.1007/s12311-016-0840-7. PMID 28032321.
- ↑ "Alternative Alzheimer's Treatments Offering Hope - 84% of the subjects surveyed rated their psychological well-being after the TPS treatment as medium to good". 11 April 2023. https://hitoshin.com/blog/alternative-alzheimers-treatments-offering-hope/.
- ↑ "Rethinking the role of sham TMS". Frontiers in Psychology 6: 210. 2015. doi:10.3389/fpsyg.2015.00210. PMID 25767458.
- ↑ "Challenges of proper placebo control for non-invasive brain stimulation in clinical and experimental applications". The European Journal of Neuroscience 38 (7): 2973–2977. October 2013. doi:10.1111/ejn.12307. PMID 23869660. https://zenodo.org/record/3436292.
- ↑ "Neurostimulation therapies in depression: a review of new modalities". Acta Psychiatrica Scandinavica 116 (3): 174–181. September 2007. doi:10.1111/j.1600-0447.2007.01033.x. PMID 17655558.
- ↑ "Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis". PLOS ONE 4 (3): e4824. 2009. doi:10.1371/journal.pone.0004824. PMID 19293925. Bibcode: 2009PLoSO...4.4824B.
- ↑ "Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: a systematic review". The World Journal of Biological Psychiatry 12 (4): 240–248. June 2011. doi:10.3109/15622975.2010.541281. PMID 21426265.
- ↑ "Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps". Pharmacology & Therapeutics 133 (1): 98–107. January 2012. doi:10.1016/j.pharmthera.2011.09.003. PMID 21924290.
- ↑ "FDA clears Nexstim´s Navigated Brain Stimulation for non-invasive cortical mapping prior to neurosurgery – Archive – Press Releases". nexstim.com. http://www.nexstim.com/news-and-events/press-releases/press-releases-archive/fda-clears-nexstim-s-navigated-brain-stimulation-for-non-invasive-cortical-mapping-prior-to-neurosurgery/.
- ↑ "Nexstim Announces FDA Clearance for NexSpeech® – Enabling Noninvasive Speech Mapping Prior to Neurosurgery". businesswire.com. 11 June 2012. http://www.businesswire.com/news/home/20120611005730/en/Nexstim-Announces-FDA-Clearance-NexSpeech%C2%AE-%E2%80%93-Enabling#.VGm4MGNjmnw/.
- ↑ Information about Mental Illness and the Brain. National Institutes of Health (US). 2007. https://www.ncbi.nlm.nih.gov/books/NBK20369/.
- ↑ In addition, the World Health Organization reports that the number of people living with depression has increased nearly 20 percent since 2005.
- ↑ Michael Drues, for Med Device Online. 5 February 2014 Secrets Of The De Novo Pathway, Part 1: Why Aren't More Device Makers Using It?
- ↑ "Neurostimulation for Treatment of Migraine and Cluster Headache". Pain Medicine 16 (9): 1827–1834. September 2015. doi:10.1111/pme.12792. PMID 26177612.
- ↑ ""Depression: let's talk" says WHO, as depression tops list of causes of ill health" (in en). https://www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health.
- ↑ "Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice". Depress Anxiety 29 (7): 587–96. July 2012. doi:10.1002/da.21969. PMID 22689344.
- ↑ Rodriguez-Martin, José Luis; Barbanoj, José Manuel; Schlaepfer, Te; Clos, Susana SC; Pérez, V; Kulisevsky, J; Gironell, A (22 April 2002). "Transcranial magnetic stimulation for treating depression" (in en). Cochrane Database of Systematic Reviews 2018 (11). doi:10.1002/14651858.CD003493. PMC 6516872. https://www.cochrane.org/CD003493/DEPRESSN_transcranial-magnetic-stimulation-tms-depression. Retrieved 11 December 2023.
- ↑ Hendon, Jessica (2002). "Transcranial magnetic stimulation for treating depression" (in en). doi:10.1002/14651858.cd003493/detailed-comment/en?messageid=412823984. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003493/detailed-comment/en?messageId=412823984.
- ↑ "FDA permits marketing of transcranial magnetic stimulation for treatment of obsessive compulsive disorder". 2020-02-20. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm617244.htm.
- ↑ "MagVenture receives FDA clearance for OCD | Clinical TMS Society". https://www.clinicaltmssociety.org/news/2020-08/magventure-receives-fda-clearance-ocd-0.
- ↑ "FDA clears OCD motor threshold cap for transcranial magnetic stimulation system" (in en). https://www.healio.com/news/neurology/20230613/fda-clears-ocd-motor-threshold-cap-for-transcranial-magnetic-stimulation-system.
- ↑ Jump up to: 79.0 79.1 79.2 "Brainsway reports positive Deep TMS system trial data for OCD". Medical Device Network (Medicaldevice-network). September 6, 2013. http://www.medicaldevice-network.com/news/newsbrainsway-reports-positive-deep-tms-system-trial-data-ocd.
- ↑ Jump up to: 80.0 80.1 80.2 80.3 80.4 "Brainsway's Deep TMS EU Cleared for Neuropathic Chronic Pain". medGadget. July 3, 2012. http://www.medgadget.com/2012/07/brainsways-deep-tms-eu-cleared-for-neuropathic-chronic-pain.html.
- ↑ "H-coil repetitive transcranial magnetic stimulation for treatment of temporal lobe epilepsy: A case report". Epilepsy & Behavior Case Reports 5 (Supplement C): 52–56. 2016-01-01. doi:10.1016/j.ebcr.2016.03.001. PMID 27114902.
- ↑ "Retrospective Evaluation of Deep Transcranial Magnetic Stimulation as Add-On Treatment for Parkinson's Disease". Frontiers in Neurology 6: 210. 2015-10-26. doi:10.3389/fneur.2015.00210. PMID 26579065.
- ↑ Petrosino, Nicholas J.; Cosmo, Camila; Berlow, Yosef A.; Zandvakili, Amin; van ’t Wout-Frank, Mascha; Philip, Noah S.. "Transcranial magnetic stimulation for post-traumatic stress disorder". Therapeutic Advances in Psychopharmacology. doi:10.1177/20451253211049921. PMID 34733479. PMC 8558793. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8558793/. Retrieved January 16, 2024.
- ↑ "Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS)". NeuroImage 85 Pt 3 (3): 961–970. January 2014. doi:10.1016/j.neuroimage.2013.06.007. PMID 23770409.
- ↑ NICE About NICE: What we do
- ↑ Jump up to: 86.0 86.1 "Transcranial magnetic stimulation for severe depression (IPG242)". London: National Institute for Health and Clinical Excellence. 2011-03-04. https://www.nice.org.uk/guidance/ipg242.
- ↑ "Transcranial magnetic stimulation for treating and preventing migraine". London: National Institute for Health and Clinical Excellence. January 2014. https://www.nice.org.uk/guidance/ipg477.
- ↑ "Repetitive transcranial magnetic stimulation for depression". 16 December 2015. https://www.nice.org.uk/guidance/ipg542.
- ↑ "Medical Policy: Transcranial Magnetic Stimulation for Depression and Other Neuropsychiatric Disorders". Policy No. BEH.00002. Anthem, Inc.. 2013-04-16. http://www.anthem.com/ca/medicalpolicies/policies/mp_pw_a047769.htm.
- ↑ Health Net (March 2012). "National Medical Policy: Transcranial Magnetic Stimulation". Policy Number NMP 508. Health Net. https://www.healthnet.com/static/general/unprotected/pdfs/national/policies/Transcranial_Magnetic_Stimulation_Mar_12.pdf.
- ↑ "Medical Policy Manual". Section IV.67. Blue Cross Blue Shield of Nebraska. 2011-05-18. https://www.nebraskablue.com/~/media/pdf/Provider/Policy%20Procedure%20Manuals/MedicalPolicies.pdf.
- ↑ "Medical Coverage Policy: Transcranial Magnetic Stimulation for Treatment of Depression and Other Psychiatric/Neurologic Disorders". Blue Cross Blue Shield of Rhode Island. 2012-05-15. https://www.bcbsri.com/sites/default/files/polices/TranscranialMagneticStimulationasaTreatmentofDepressionandOtherPsychiatricNeurologicDisorders_0.pdf.
- ↑ UnitedHealthcare (2013-12-01). "Transcranial Magnetic Stimulation". UnitedHealthCare. p. 2. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Transcranial_Magnetic_Stimulation.pdf.
- ↑ Aetna (2013-10-11). "Clinical Policy Bulletin: Transcranial Magnetic Stimulation and Cranial Electrical Stimulation". Number 0469. Aetna. http://www.aetna.com/cpb/medical/data/400_499/0469.html.
- ↑ Cigna (2013-01-15). "Cigna Medical Coverage Policy: Transcranial Magnetic Stimulation". Coverage Policy Number 0383. Cigna. http://www.cigna.com/assets/docs/health-care-professionals/coverage_positions/mm_0383_coveragepositioncriteria_transcranial_magnetic_stimulation.pdf.
- ↑ Regence (2013-06-01). "Medical Policy: Transcranial Magnetic Stimulation as a Treatment of Depression and Other Disorders". Policy No. 17. Regence. http://blue.regence.com/trgmedpol/medicine/med148.pdf.
- ↑ "Medicare Administrative Contractors". Centers for Medicare and Medicaid Services. 2013-07-10. https://www.cms.gov/Medicare/Medicare-Contracting/Medicare-Administrative-Contractors/MedicareAdministrativeContractors.html.
- ↑ NHIC, Corp. (2013-10-24). "Local Coverage Determination (LCD) for Repetitive Transcranial Magnetic Stimulation (rTMS) (L32228)". Centers for Medicare and Medicaid Services. http://coverage.cms.fu.com/mcd_archive/viewlcd.asp?lcd_id=32228&lcd_version=5&basket=lcd%3A32228%3A5%3ARepetitive+Transcranial+Magnetic+Stimulation+%28rTMS%29%3AMAC+%2D+Part+B%3ANHIC%7C%7C+Corp%2E+%2814202%29%3A.
- ↑ "Important Treatment Option for Depression Receives Medicare Coverage". Press Release. PBN.com: Providence Business News. 2012-03-30. http://www.pbn.com/Important-Treatment-Option-for-Depression-Receives-Medicare-Coverage,66462.
- ↑ The Institute for Clinical and Economic Review (June 2012). "Coverage Policy Analysis: Repetitive Transcranial Magnetic Stimulation (rTMS)". The New England Comparative Effectiveness Public Advisory Council (CEPAC). http://cepac.icer-review.org/wp-content/uploads/2012/07/rTMS-Coverage-Policy-Analysis.pdf.
- ↑ "Transcranial Magnetic Stimulation Cites Influence of New England Comparative Effectiveness Public Advisory Council (CEPAC)". Berlin, Vermont: Central Vermont Medical Center. 2012-02-06. http://www.cvmc.org/news/2012-theresa-fama-cepac.
- ↑ National Government Services, Inc. (2013-10-25). "Local Coverage Determination (LCD): Transcranial Magnetic Stimulation (L32038)". Centers for Medicare and Medicaid Services. http://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=32038&ContrId=178&&bc=IAAAABAAAAAAAA%3d%3d&.
- ↑ Novitas Solutions, Inc. (2013-12-04). "LCD L32752 – Transcranial Magnetic Stimulation for Depression". Contractor's Determination Number L32752. Centers for Medicare and Medicaid Services. http://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=32752&ContrId=259&ver=15&ContrVer=1&DocID=L32752&SearchType=Advanced&bc=IAAAAAgAAAAAAA%3d%3d&l.
- ↑ Novitas Solutions, Inc. (2013-12-05). "LCD L33660 – Transcranial Magnetic Stimulation (TMS) for the Treatment of Depression". Contractor's Determination Number L33660. Centers for Medicare and Medicaid Services. http://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33660&ContrId=259&bc=IAAAAAgAAAAAAA%3d%3d&.
 |