Physics:Ethenium
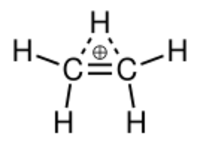
In chemistry, ethenium, protonated ethylene or ethyl cation is a positive ion with the formula C2H+5. It can be viewed as a molecule of ethylene (C2H4) with one added proton (H+), or a molecule of ethane (C2H6) minus one hydride ion (H−). It is a carbocation; more specifically, a nonclassical carbocation.
Preparation
Ethenium has been observed in rarefied gases subjected to radiation.[1] Another preparation method is to react certain proton donors such as H+3, HeH+, N2H+, and N2OH+ with ethane at ambient temperature and pressures below 1 mmHg. (Other donors such as CH+5 and HCO+ form ethanium preferably to ethenium.)[2]
At room temperature and in a rarefied methane atmosphere, ethanium slowly dissociates to ethenium and H2. The reaction is much faster at 90 °C.[1]
Stability and reactions
Contrary to some earlier reports, ethenium was found to be largely unreactive towards neutral methane at ambient temperature and low pressure (on the order of 1 mmHg), even though the reaction yielding sec-C3H+7 and H2 is believed to be exothermic.[3]
Structure
The structure of ethenium's ground state was in dispute for many years, but it was eventually agreed to be a non-classical structure, with the two carbon atoms and one of the hydrogen atoms forming a three-center two-electron bond. Calculations have shown that higher homologues, like the propyl and n-butyl cations also have bridged structures. Generally speaking, bridging appears to be a common means by which 1° alkyl carbocations achieve additional stabilization. Consequently, true 1° carbocations (with a classical structure) may be rare or nonexistent.[clarification needed]
References
- ↑ 1.0 1.1 Margaret French and Paul Kebarle (1975), "Pyrolysis of C2H+7 and other ion-molecule reactions in methane containing traces of ethane". Canadian Journal of Chemistry, volume 53, pages 2268-2274. doi:10.1139/v75-318
- ↑ G. I. Mackay, H. I. Schiff, D. K. Bohme (1981), "A room-temperature study of the kinetics and energetics for the protonation of ethane" Canadian Journal of Chemistry, volume 59, issue 12,pages 1771-1778. doi:10.1139/v81-265
- ↑ F. H. Field , M. S. B. Munson (1965), "Reactions of gaseous ions. XIV. Mass spectrometric studies of methane at pressures to 2 Torr". Journal of the American Chemical Society, volume 87, issue 15, pages 3289–3294 doi:10.1021/ja01093a001
 |
