Software:Aqion
| Initial release | January 1, 2012 |
|---|---|
| Stable release | version 8.4
/ Dec 2023 |
| Written in | C++ |
| Operating system | Windows |
| Size | 5 MB |
| Available in | English, German |
| Website | aqion.de |
Aqion is a hydrochemistry software tool. It bridges the gap between scientific software (such like PhreeqC[1]) and the calculation/handling of "simple" water-related tasks in daily routine practice. The software aqion is free for private users, education and companies.
Motivation & history
First. Most of the hydrochemical software is designed for experts and scientists. In order to flatten the steep learning curve aqion provides an introduction to fundamental water-related topics in form of a "chemical pocket calculator".
Second. The program mediates between two terminological concepts: The calculations are performed in the "scientific realm" of thermodynamics (activities, speciation, log K values, ionic strength, etc.). Then, the output is translated into the "language" of common use: molar and mass concentrations, alkalinity, buffer capacities, water hardness, conductivity and others.
History. Version 1.0 was released in January 2012 (after a half-year test run in 2011). The project is active with 1-2 updates per month.
Features
- Validates aqueous solutions (charge balance error, parameter adjustment)
- Calculates physico-chemical parameters: alkalinity, buffer capacities (ANC, BNC), water hardness, ionic strength
- Calculates aqueous speciation and complexation
- Calculates pH of solutions after addition of chemicals (acids, bases, salts)
- Calculates the calcite-carbonate system (closed/open CO
2 system, Langelier Saturation Index) - Calculates mineral dissolution, precipitation, and saturation indices
- Calculates mixing of two waters
- Calculates reduction-oxidation (redox) reactions
- Plots titration curves
Fields of application
- Water analysis and water quality
- Geochemical modeling (in simplest form)
- Education
Limits of application
- only inorganic species (no organic chemistry)
- only equilibrium thermodynamics (no chemical kinetics)
- only aqueous solutions with ionic strength ≤ 0.7 mol/L[2] (no brines)
Basic algorithm & numerical solver
There are two fundamental approaches in hydrochemistry: Law of mass action (LMA) and Gibbs energy minimization (GEM).[3] The program aqion belongs to the category LMA approach. In a nutshell: A system of NB independent basis components j (i.e. primary species), that combines to form NS secondary species i, is represented by a set of mass-action and mass-balance equations:
(1) mass action law: with i = 1 ... NS
(2) mass balance law: with j = 1 ... NB
where Ki is the equilibrium constant of formation of the secondary species i, and νi,j represents the stoichiometric coefficient of basis species j in secondary species i (the values of νj,i can be positive or negative). Here, activities ai are symbolized by curly brackets {i} while concentrations ci by rectangular brackets [i]. Both quantities are related by the
(3) activity correction:
with γi as the activity coefficient calculated by the Debye–Hückel equation and/or Davies equation. Inserting Eq.(1) into Eq.(2) yields a nonlinear polynomial function fj for the j-th basis species:
(4)
which is the objective function of the Newton–Raphson method.
To solve Eq.(4) aqion adopts the numerical solver from the open-source software PhreeqC.[1][4] The equilibrium constants Ki are taken from the thermodynamic database wateq4f. [5]
Examples, test & verification
The software aqion is shipped with a set of example solutions (input waters) and a tutorial how to attack typical water-related problems (online-manual with about 40 examples). More examples and exercises for testing and re-run can be found in classical textbooks of hydrochemistry.[6][7][8]
The program was verified by benchmark tests of specific industry standards.[9]
Screenshots
-
Input panel for chemical elements
-
Output of pH calculator (example)
-
Calculated parameters of the calcite carbonate system
-
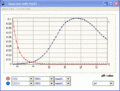
titration curves (example: addition of NaOH to a given input water)
References
- ↑ 1.0 1.1 Parkhurst, D.L. and C.A.J. Appelo: User's Guide to PHREEQC (version 2), a computer program for speciation, batch-reaction, one-dimensional transport and inverse geochemical calculations. USGS Water-Resources Investigations Report 99-4259, 1999
- ↑ Note: The upper limit is sea water.
- ↑ http://www.kristall.uni-frankfurt.de/media/handouts/GEM-lecture.PDF[yes|permanent dead link|dead link}}]
- ↑ Remark: To keep things apart, the numerical solver of PhreeqC is outsourced from aqion.exe into a separate DLL.
- ↑ Ball J. W. and D. K. Nordstrom: WATEQ4F – "User’s manual with revised thermodynamic data base and test cases for calculating speciation of major, trace and redox elements in natural waters", USGS Open-File Report 90-129, 185 p, 1991.
- ↑ Stumm, W. and J. J. Morgan: Aquatic Chemistry, Chemical Equilibria and Rates in Natural Waters (3rd ed.), John Wiley & Sons, Inc., New York, 1996, ISBN 978-0471511854
- ↑ Morel, F. M. M. and J. G. Hering: Principles and Applications of Aquatic Chemistry (2nd ed.), John Wiley, New York, 1993, ISBN 978-0471548966
- ↑ Appelo, C. A. J. and D. Postma: Geochemistry, Groundwater, and Pollution. Taylor & Francis, 2005, ISBN 978-0415364287
- ↑ DIN 38404-10: German standard methods for the examination of water, waste water and sludge - Physical and physicochemical parameters (group C) - Determination of calcite saturation of water (C 10)
External links
- PHREEQC
- Online calculator for pH, aqueous speciation, saturation indices, alkalinity, EC
 |