Chemistry:PHCCC: Difference between revisions
From HandWiki
(over-write) |
(No difference)
|
Latest revision as of 08:36, 8 February 2024
Short description: Chemical compound
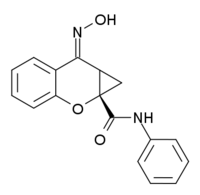 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C17H14N2O3 |
| Molar mass | 294.310 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
PHCCC is a research drug which acts as a glutamate receptor ligand, particularly being a positive allosteric modulator at the mGluR4 subtype,[1] as well as an agonist at mGluR6.[2] It has anxiolytic effects in animal studies.[3] PHCCC and similar drugs have been suggested as novel treatments for Parkinson's disease.[4]
See also
References
- ↑ "(−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection". Neuropharmacology 45 (7): 895–906. December 2003. doi:10.1016/S0028-3908(03)00271-5. PMID 14573382.
- ↑ "The mGlu(4) receptor allosteric modulator N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide acts as a direct agonist at mGlu(6) receptors". European Journal of Pharmacology 589 (1–3): 49–52. July 2008. doi:10.1016/j.ejphar.2008.06.054. PMID 18593581.
- ↑ "Anxiolytic-like effects of PHCCC, an allosteric modulator of mGlu4 receptors, in rats". European Journal of Pharmacology 498 (1–3): 153–6. September 2004. doi:10.1016/j.ejphar.2004.07.001. PMID 15363989.
- ↑ "Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4". Molecular Pharmacology 74 (5): 1345–58. November 2008. doi:10.1124/mol.108.049551. PMID 18664603.
 |

