Chemistry:Fluorosulfite
| |
| Names | |
|---|---|
| Other names
Sulfurofluoridoite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FO2S−1 | |
| Molar mass | 83.06 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
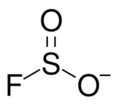
Fluorosulfite is an ion with the formula SO2F−. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier.[1]
Organic compounds with the name "fluorosulfite" contain the group -OS(O)F.[2]
Preparation
[((CH3)2N)3SO][SO2F] can be prepared from OSF4 and Me3SiNMe2.[3] Alkali metal fluorosulfites can be made by soaking the metal fluoride in liquid sulfur dioxide for a few days.[4] β-CsSO2F converts to α-CsSO2F when heated to 110 °C for a couple of days but remains stable below 50 °C.[4]
Properties
The fluorosulfite ion is tetrahedral, with sulfur at the top. The oxygen to sulfur bonds are 147.8 pm and the fluorine to sulfur bond is >169.0 pm long.[4] In solid ionic fluorosulfites, the ion is not fixed in orientation and continuously turns around resulting in dynamic disorder. At room temperature this turning rate is from 2×105 to 107 Hz. When cooled the rate of rotation slows, and can be frozen in place, resulting in static disorder.[4]
Fluorosulfite is isoelectronic with chloryl fluoride (ClO2F) and in compounds it resembles chlorate (ClO3−).[4]
The heat of formation from fluoride (F−) and sulfur dioxide (SO2) is 50 kcal mol−1.[5]
Reactions
Fluorosulfites can react with chlorophosphazenes to make fluorophosphazenes:[6]
- (NPCl2)n + 2n KSO2F → (NPF2)n + 2n KCl + 2nSO2 n=3 or 4
Related
Fluorosulfite is in the category of halosulfite ions which include chlorosulfite, bromosulfite and iodosulfite.[7] Related ions include cyanosulfite SO2CN−.[8]
List
| name | formula | weight | system | space group | unit cell | volume | density | properties | ref |
|---|---|---|---|---|---|---|---|---|---|
| NO[SO2F] | [1] | ||||||||
| 2-chloro-1,3-diisopropyl-4,5-dimethylimidazolium fluorosulfite | C11H20FClN2O2S | [9] | |||||||
| 2-fluoro-1,3-diisopropyl-4,5-dimethylimidazolium fluorosulfite | C11H20F2N2O2S | P21/c | a=15.097 b=13.406 c=15.776 β=113.54 Z=8 | 2927 | [9] | ||||
| (difluoromethyl)triphenylphosphonium fluorosulfite | [Ph3PCF2H][SO2F] | white, melt ~240 °C | [10] | ||||||
| tetramethylammonium fluorosulfite | [(CH3)4N][SO2F] | 157.21 | orthorhombic | Pbca | a=11.520 b=11.505 c=11.627 Z=8 | 1541.0 | 1.355 | [3] | |
| tris-dimethylamino sulfonium fluorosulfite | [((CH3)2N)3S][SO2F] | 247.35 | orthorhombic | Pnma | a=14.690 b=11.174 c=7.3340 Z=4 | 1203.8 | 1.365 | [3] | |
| tris-dimethylamino sulfoxonium fluorosulfite | [((CH3)2N)3SO][SO2F] | 263.65 | orthorhombic | Pna21 | a=21.850 b=6.733 c=8.194 Z=4 | 1205.5 | 1.451 | [3] | |
| potassium fluorosulfite | KSO2F | 122.16 | monoclinic | P21/m | a=6.9725 b=5.6713 c=4.6653 β=107.702° Z=2 | 175.75 | 2.308 | [4] | |
| rubidium fluorosulfite | RbSO2F | monoclinic | P21/m | a=7.175 b=5.859 c=4.8416 β=107.18° Z=2 | 194.45 | 2.878 | [4] | ||
| alpha caesium fluorosulfite | α-CsSO2F | orthorhombic | Pnma | a=7.9098 b=6.6607 c=7.9893 z=4 | 420.91 | 3.408 | [4] | ||
| beta caesium fluorosulfite | β-CsSO2F | rhombohedral | R3m | a=6.5922 c=8.0050 z=3 | 301.27 | 3.571 | [4] | ||
References
- ↑ 1.0 1.1 Seel, F.; Meier, H. (December 1953). "Die Chemie des Nitrosyl-Ions. IX. Über den Chemismus des Bleikammerverfahrens" (in de). Zeitschrift für anorganische und allgemeine Chemie 274 (4–5): 197–222. doi:10.1002/zaac.19532740404. ISSN 0044-2313.
- ↑ Baasner, Bernd (2014) (in en). Houben-Weyl Methods of Organic Chemistry Vol. E 10a, 4th Edition Supplement: Organo-Fluorine Compounds - Fluorinating Agents and Their Application in Organic Synthesis. Georg Thieme Verlag. pp. 332–334. ISBN 978-3-13-181544-6. https://books.google.com/books?id=6y6GAwAAQBAJ&pg=PA332.
- ↑ 3.0 3.1 3.2 3.3 Lork, Enno; Mews, Rüdiger; Viets, Detlef; Watson, Paul G.; Borrmann, Tobias; Vij, Ashwani; Boatz, Jerry A.; Christe, Karl O. (March 2001). "Structure of the SO 2 F - Anion, a Problem Case 1" (in en). Inorganic Chemistry 40 (6): 1303–1311. doi:10.1021/ic000616p. ISSN 0020-1669. PMID 11300833.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Kessler, Ulrich; van Wüllen, Leo; Jansen, Martin (2001-12-01). "Structure of the Fluorosulfite Anion: Rotational Disorder of SO2F– in the Alkali Metal Fluorosulfites and Crystal Structures of α- and β-CsSO2F". Inorganic Chemistry 40 (27): 7040–7046. doi:10.1021/ic010303+. ISSN 0020-1669. PMID 11754288.
- ↑ Maulitz, Andreas H.; Boese, Roland; Kuhn, Norbert (April 1995). "Ab initio studies on halosulfite ions". Journal of Molecular Structure: THEOCHEM 333 (3): 227–232. doi:10.1016/0166-1280(94)03955-K.
- ↑ Allcock, H. (2012) (in en). Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems. Elsevier. p. 207. ISBN 978-0-323-14751-4. https://books.google.com/books?id=UQ5cY4vN9NYC&pg=PA207.
- ↑ Robertson, Katherine N.; Land, Michael A.; Murphy, Luke J.; Doyle, Kirstin A.; Clyburne, Jason A. C. (2018-07-20). "Sulfur dioxide–halide ion complexes: a crystallographic investigation of bonding". Acta Crystallographica Section A 74 (a1): a341. doi:10.1107/S0108767318096599. ISSN 2053-2733. http://scripts.iucr.org/cgi-bin/paper?S0108767318096599.
- ↑ Kornath, Andreas; Blecher, Oliver; Ludwig, Ralf (April 1999). "Synthesis and Characterization of Tetramethylammonium Cyanosulfite, (CH3)4N+SO2CN–" (in en). Journal of the American Chemical Society 121 (16): 4019–4022. doi:10.1021/ja9833422. ISSN 0002-7863.
- ↑ 9.0 9.1 Kuhn, Norbert; Bohnen, Hans; Fahl, Joanna; Bläser, Dieter; Boese, Roland (December 1996). "Derivate des Imidazols, XIX. Koordination oder Reduktion? Zur Reaktion von 1,3-Diisopropyl-4,5-dimethylimidazol-2-yliden mit Schwefelhalogeniden und Schwefeloxidhalogeniden" (in de). Chemische Berichte 129 (12): 1579–1586. doi:10.1002/cber.19961291228.
- ↑ Zhu, Shi-Zheng; Huang, Qi-Chen; Wu, Kuang (September 1994). "Synthesis and Structure of (Difluoromethyl)triphenylphosphonium Fluorosulfite, Evidence for Formation of Difluorosulfene as an Intermediate" (in en). Inorganic Chemistry 33 (20): 4584–4585. doi:10.1021/ic00098a028. ISSN 0020-1669.
 |

