Chemistry:Isoelectronicity
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, CO, NO+, and N2 are isoelectronic, while CH3COCH3 and CH3N=NCH3 are not.[1]
This definition is sometimes termed valence isoelectronicity. Definitions can sometimes be not as strict, sometimes requiring identity of the total electron count and with it the entire electronic configuration.[2] More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.[3]
The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic species can be expected to show useful consistency and predictability in their properties, so identifying a compound as isoelectronic with one already characterised offers clues to possible properties and reactions. Differences in properties such as electronegativity of the atoms in isolelectronic species can affect reactivity.
In quantum mechanics, hydrogen-like atoms are ions with only one electron such as Li2+. These ions would be described as being isoelectronic with hydrogen.
Examples
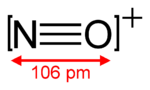
The N atom and the O+ ion are isoelectronic because each has five valence electrons, or more accurately an electronic configuration of [He] 2s2 2p3.
Similarly, the cations K+, Ca2+, and Sc3+ and the anions Cl−, S2−, and P3− are all isoelectronic with the Ar atom.
CO, CN−, N2, and NO+ are isoelectronic because each has two atoms triple bonded together, and due to the charge have analogous electronic configurations (N− is identical in electronic configuration to O so CO is identical electronically to CN−).
Molecular orbital diagrams best illustrate isoelectronicity in diatomic molecules, showing how atomic orbital mixing in isoelectronic species results in identical orbital combination, and thus also bonding.
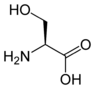
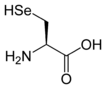
More complex molecules can be polyatomic also. For example, the amino acids serine, cysteine, and selenocysteine are all isoelectronic to each other. They differ by which specific chalcogen is present at one location in the side-chain.
CH3COCH3 (acetone) and CH3N2CH3 (azomethane) are not isoelectronic. They do have the same number of electrons but they do not have the same structure.
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isoelectronic". doi:10.1351/goldbook.I03276
- ↑ Isoelectronic Configurations iun.edu
- ↑ A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) Advances in Organometallic Chemistry Vol. 30 (1990), Chapter 5 (at p. 190) google books link
 |