Chemistry:C-1027
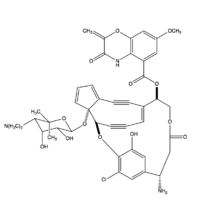
| |
| Names | |
|---|---|
| IUPAC name
(3R,4R,14R,19S)-22-chloro-4-{[(2S,3R,4R,5S)-5-(dimethylamino)-3,4-dihydroxy-6,6-dimethyloxan-2-yl]oxy}-23-hydroxy-14-(3-hydroxy-7-methoxy-2-methylidene-2H-1,4-benzoxazine-5-carbonyloxy)-17-oxo-2,16-dioxapentacyclo[18.2.2.1⁹,¹³.0³,¹⁰.0⁴,⁸]pentacosa-1(22),5,7,9,11,13(25),20,23-octaen-19-aminium
| |
| Other names
Lidamycin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| |
| |
| Properties | |
| C43H42ClN3O13 | |
| Molar mass | 844.267 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
C-1027 or Lidamycin is an enediyne antitumor antibiotic.[2] It was first isolated as an apoprotein-chromophore complex.[3] It shows antibiotic activity against most Gram-positive bacteria.[4] It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.
C-1027’s chromophore contains a nine-membered enediyne that is responsible for most of the molecule’s biological activity.[4] Unlike other enediynes, this molecule contains no triggering mechanism. It is already primed to undergo the cycloaromatization reaction without external activation to produce the toxic 1,4-benzenoid diradical species. C-1027 can induce oxygen-independent interstrand DNA crosslinks in addition to the oxygen-dependent single- and double-stranded DNA breaks typically generated by other enediynes. This unique oxygen-independent mechanism suggests that C-1027 may be effective against hypoxic tumor cells.[5]
C-1027 shows promise as an anticancer drug and is currently undergoing phase II clinical trials in China,[6] with a 30% success rate.[7] It can induce apoptosis in many cancer cells and recent studies have indicated that it induces unusual DNA damage responses to double-strand breaks, including altering cell cycle progression and inducing chromosomal aberrations.[2]
References
- ↑ Pubchem. "Lidamycin". https://pubchem.ncbi.nlm.nih.gov/compound/9962646#section=Substances-by-Category.
- ↑ 2.0 2.1 "Lidamycin shows highly potent cytotoxic to myeloma cells and inhibits tumor growth in mice". Acta Pharmacologica Sinica 30 (7): 1025–32. July 2009. doi:10.1038/aps.2009.75. PMID 19575006.
- ↑ "Complexity and simplicity in the biosynthesis of enediyne natural products". Natural Product Reports 27 (4): 499–528. April 2010. doi:10.1039/b908165h. PMID 20336235.
- ↑ 4.0 4.1 "C1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage". Biochemistry 33 (19): 5947–54. May 1994. PMID 8180224.
- ↑ "Improvement of the enediyne antitumor antibiotic C-1027 production by manipulating its biosynthetic pathway regulation in Streptomyces globisporus". Journal of Natural Products 74 (3): 420–4. March 2011. doi:10.1021/np100825y. PMID 21250756.
- ↑ "Draft genome sequence of Streptomyces globisporus C-1027, which produces an antitumor antibiotic consisting of a nine-membered enediyne with a chromoprotein". Journal of Bacteriology 194 (15): 4144. August 2012. doi:10.1128/JB.00797-12. PMID 22815456.
- ↑ "Enediynes: Exploration of microbial genomics to discover new anticancer drug leads". Bioorganic & Medicinal Chemistry Letters 25 (1): 9–15. January 2015. doi:10.1016/j.bmcl.2014.11.019. PMID 25434000.
 |


