Chemistry:Maximiscin
From HandWiki
Revision as of 15:38, 17 July 2022 by imported>CodeMe (url)
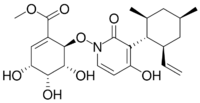
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (3R,4R,5R,6R)-6-({3-[(1R,2S,4R,6S)-2-ethenyl-4,6-dimethylcyclohexyl]-4-hydroxy-2-oxopyridin-1(2H)-yl}oxy)-3,4,5-trihydroxycyclohex-1-ene-1-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C23H31NO8 | |
| Molar mass | 449.500 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Maximiscin is a polyketide-shikimate chemical compound isolated from Tolypocladium that shows tumor growth suppression in an animal model.[1] The discovery of maximiscin was the result of a citizen scientist crowdsourcing project by the University of Oklahoma.[2] The soil sample which yielded maximiscin was sent by a woman from Salcha, Alaska.[3]
References
- ↑ Du, L; Robles, AJ; King, JB; Powell, DR; Miller, AN; Mooberry, SL; Cichewicz, RH (2013). "Maximiscin, a Novel Shikimate-Polyketide-NRPS Hybrid Metabolite Obtained from Tolypocladium Sp. With Potent Antitumor Activities". Planta Medica 79 (10). doi:10.1055/s-0033-1348528.
- ↑ Du, L; Robles, AJ; King, JB; Powell, DR; Miller, AN; Mooberry, SL; Cichewicz, RH (2013). "Crowdsourcing Natural Products Discovery to Access Uncharted Dimensions of Fungal Metabolite Diversity". Angewandte Chemie International Edition in English 53 (3): 804–9. doi:10.1002/anie.201306549. PMID 24285637.
- ↑ Julianne Wyrick (December 5, 2013). "Crowdsourcing unearths promising anticancer compound". Royal Society of Chemistry. http://www.rsc.org/chemistryworld/2013/12/crowdsourcing-delivers-promising-anticancer-fungi-compound.
 |

