Chemistry:Calmagite
From HandWiki
Revision as of 08:06, 28 June 2021 by imported>Steve Marsio (add)
| |
| |
| Names | |
|---|---|
| IUPAC name
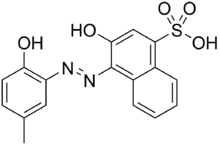
3-Hydroxy-4-[(2-hydroxy-5-methylphenyl)azo]-1-naphthalenesulfonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H14N2O5S | |
| Molar mass | 358.37 g·mol−1 |
| Appearance | Red to black crystals |
| Odor | Phenolic odor |
| Melting point | 330 °C (626 °F; 603 K) |
| Hazards | |
| Main hazards | May emit ammonia, oxides of sulfur, oxides of nitrogen, and oxides of carbon. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Calmagite is a complexometric indicator used in analytical chemistry to identify the presence of metal ions in solution. As with other metal ion indicators calmagite will change color when it is bound to an ion. Calmagite will be wine red when it is bound to a metal ion and may be blue, red, or orange when it is not bound to a metal ion. Calmagite is often used in conjunction with EDTA, a stronger metal binding agent.[2] This chemical is also used in the quantitation of magnesium in the clinical laboratory.
References
- ↑ Calmagite at Sigma-Aldrich
- ↑ Harris, Daniel C. Quantitative Chemical Analysis, W.H. Freeman and Company ,2007. ISBN:0-7167-7041-5
 |

