Chemistry:Triacetylmethane
From HandWiki
| |
| Names | |
|---|---|
| Other names
3-acetyl-2,4-pentanedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C7H10O3 | |
| Molar mass | 142.154 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.0591 g/cm3 |
| Boiling point | 96–97 °C (205–207 °F; 369–370 K) 15 torr |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264+265Script error: No such module "Preview warning".Category:GHS errors, P280, P305+351+338, P337+317Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
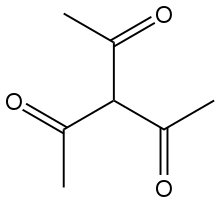
Triacetylmethane is the organic compound with the formula HC(C(O)CH
3)
3. It is a colorless liquid that is soluble in organic solvents and in alkaline water. It readily forms an enolate.[2][3] The enolate forms a variety of metal complexes related to the metal acetylacetonates.[4]
References
- ↑ "Triacetylmethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/69950#section=Safety-and-Hazards.
- ↑ Arnett, Edward M.; Maroldo, Stephen G.; Schilling, Steven L.; Harrelson, John A. (1984). "Ion pairing and reactivity of enolate anions. 5. Thermodynamics of ionization of .beta.-di- and tricarbonyl compounds in dimethyl sulfoxide solution and ion pairing of their alkali salts". Journal of the American Chemical Society 106 (22): 6759–6767. doi:10.1021/ja00334a049.
- ↑ Yoshida, Z.; Ogoshi, H.; Tokumitsu, T. (1970). "Intramolecular Hydrogen Bond in Enol Form of 3-Substituted-2,4-Pentanedione". Tetrahedron 26 (24): 5691–5697. doi:10.1016/0040-4020(70)80005-9.
- ↑ Basato, Marino; Caneva, Elisabetta; Tubaro, Cristina; Veronese, Augusto Cesare (2009). "Coordinating Properties of the Anionic Ligand (MeCO)2C(−)C(X)Me (X=O or NH) Toward Transition Metal(II) Centers". Inorganica Chimica Acta 362 (8): 2551–2555. doi:10.1016/j.ica.2008.11.017.
 |

