Chemistry:Cycloprop-2-ene carboxylic acid
From HandWiki
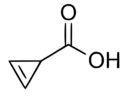
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cycloprop-2-ene-1-carboxylic acid[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H4O2 | |
| Molar mass | 84.074 g·mol−1 |
| Melting point | 40–41 °C (104–106 °F; 313–314 K)[2] |
| log P | -0.816 |
| Acidity (pKa) | 4.246 |
| Basicity (pKb) | 9.751 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cycloprop-2-ene carboxylic acid is a mycotoxin found in some mushrooms such as Russula subnigricans.[3]
When ingested, the molecule is known to cause rhabdomyolysis.[3]
In mice, the oral -1">50 of this molecule is 2.5 mg/kg and poisoning is indicated by an increase in serum creatine phosphokinase activity. Polymerization via the ene reaction abolishes toxicity.[3]
3-(Cycloprop-2-en-1-oyl)oxazolidinones are a class of ‘unusually stable’ derivatives of cycloprop-2-ene carboxylic acid that have been synthesized by Fox et al. As mentioned by Fox et al, this class of ‘unusually stable’ derivatives are dienophiles when involved in a Diels-Alder reaction.[2]
References
- ↑ "NChemBio.179-comp1". The PubChem Project. USA: National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=25241629.
- ↑ 2.0 2.1 Yan, Ni; Liu, Xiaozhong; Pallerla, Mahesh K.; Fox, Joseph M. (2008-06-01). "Synthesis of Stable Derivatives of Cycloprop-2-ene Carboxylic Acid". The Journal of Organic Chemistry 73 (11): 4283–4286. doi:10.1021/jo800042w. ISSN 0022-3263. PMID 18452335.
- ↑ 3.0 3.1 3.2 Matsuura, Masanori; Saikawa, Yoko; Inui, Kosei; Nakae, Koichi; Igarashi, Masayuki; Hashimoto, Kimiko; Nakata, Masaya (2009). "Identification of the toxic trigger in mushroom poisoning". Nature Chemical Biology 5 (7): 465–7. doi:10.1038/nchembio.179. PMID 19465932.
 |

