Chemistry:Horsfiline
From HandWiki
| |
| Names | |
|---|---|
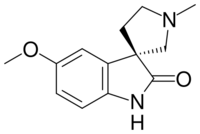
| Preferred IUPAC name
(3R)-5-Methoxy-1′-methylspiro[indole-3,3′-pyrrolidin]-2(1H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H16N2O2 | |
| Molar mass | 232.283 g·mol−1 |
| Melting point | 125 to 126 °C (257 to 259 °F; 398 to 399 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Horsfiline is an oxindole alkaloid found in the plant Horsfieldia superba,[1] which is used in traditional herbal medicine. It has analgesic effects and has been the subject of research both to produce it synthetically by convenient routes[2][3][4][5][6][7][8] and to develop analogues and derivatives which may have improved analgesic effects.[9][10]
It is a member of the spiroindolone class. Elacomine has a similar chemical structure.
References
- ↑ "An oxindole alkaloid from Horsfieldia superba". Journal of Organic Chemistry 56 (23): 6527–6530. 1991. doi:10.1021/jo00023a016.
- ↑ "Total Synthesis of (-)-Horsfiline via Asymmetric Nitroolefination". The Journal of Organic Chemistry 64 (5): 1699–1704. March 1999. doi:10.1021/jo981577q. PMID 11674239.
- ↑ "Azomethine ylide cycloaddition/reductive heterocyclization approach to oxindole alkaloids: asymmetric synthesis of (-)-horsfiline". The Journal of Organic Chemistry 66 (25): 8447–53. December 2001. doi:10.1021/jo015854w. PMID 11735524.
- ↑ "Novel phosphorus radical-based routes to horsfiline". Organic Letters 7 (15): 3287–9. July 2005. doi:10.1021/ol051095i. PMID 16018642.
- ↑ "Palladium asymmetric allylic alkylation of prochiral nucleophiles: horsfiline". Organic Letters 8 (10): 2027–30. May 2006. doi:10.1021/ol060298j. PMID 16671773.
- ↑ "Efficient enantioselective total synthesis of (-)-horsfiline". Chemistry 19 (29): 9599–605. July 2013. doi:10.1002/chem.201301008. PMID 23836402.
- ↑ "Asymmetric organocatalyzed Michael addition of nitromethane to a 2-oxoindoline-3-ylidene acetaldehyde and the three one-pot sequential synthesis of (-)-horsfiline and (-)-coerulescine". Chemistry 20 (42): 13583–8. October 2014. doi:10.1002/chem.201403932. PMID 25155110.
- ↑ "Nonstabilized Azomethine Ylides in the Mannich Reaction: Synthesis of 3,3-Disubstituted Pyrrolidines, Including Oxindole Alkaloids". The Journal of Organic Chemistry 82 (23): 12827–12833. December 2017. doi:10.1021/acs.joc.7b02193. PMID 29048900.
- ↑ "C-alkylated spiro[benzofuran-3(2H),4'-1'-methyl-piperidine-7-ols] as potent opioids: a conformation-activity study". Bioorganic & Medicinal Chemistry Letters 8 (14): 1813–8. July 1998. doi:10.1016/S0960-894X(98)00318-7. PMID 9873439.
- ↑ Alf Claesson, Britt-Marie Swahn, Odd-Geir Berge. Spirooxindole derivatives that act as analgesics. US Patent 6774132
 |

