Chemistry:BI 99179
| |
| Names | |
|---|---|
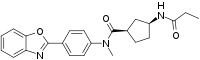
| IUPAC name
(1R,3S)-N-[4-(1,3-Benzoxazol-2-yl)phenyl]-N-methyl-3-(propanoylamino)cyclopentane-1-carboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H25N3O3 | |
| Molar mass | 391.471 g·mol−1 |
| Pharmacology | |
| Pharmacokinetics: | |
| 46% | |
| 97.6% (rat) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
BI 99179 is a selective small molecule inhibitor suitable for the in vivo validation of type 1 fatty acid synthase (FAS) as a therapeutic target for lipid metabolism-related disorders (lipid metabolism disorder) which has been discovered by Boehringer Ingelheim.[1][2]
Summary
BI 99179 is characterized by its high potency, high selectivity, and significant exposure (both peripheral and central) upon oral administration in rats.[1]
Target information
Mammalian type I FAS is a multi-enzyme protein that catalyzes fatty acid synthesis. It mediates key roles in neoplastic lipogenesis and is highly expressed in lipogenic tissues. While most tissues, except liver and adipose tissue, have low levels of FAS expression and activity, FAS is over-expressed in many cancers. Accumulating evidence suggests that it is a metabolic oncogene with an important role in tumor growth and survival, thus making it an attractive target for cancer therapy.[2][3] In addition to its role in oncology, FAS inhibition could also represent an attractive therapeutic target in obesity; both systemic and intracerebroventricular treatment of mice with FAS inhibitors led to inhibition of feeding and dramatic weight loss.[4] Moreover, inhibitors of FAS have been reported to reduce the production of sebum in sebocytes;[5] therefore, topical FAS inhibition could be a potential anti-acne approach.
FAS consists of two identical subunits, each comprising an acyl carrier protein (ACP) domain and six different catalytic domains. BI 99179 is likely to bind to the ketoacyl reductase (KR) domain (evidence: Boehringer Ingelheim enzymatic data and analogy to the published co-crystal structure of the human KR domain with GSK2194069).[6]
In vitro activity
BI 99179 inhibits the FAS enzyme isolated from HeLa cells with a half maximal inhibitory concentration (IC50) of 79 nM.
| In Vitro Activity[1][2] | |
| Probe name | BI 99179 |
| Inhibition of FASN* (IC50) [nM] | 79 |
| Inhibition of [14C]acetate incorporation§ mouse N-42 cells (IC50) [nM] | 570 |
| Inhibition of [14C]acetate incorporation§ hum. H1975 cells (IC50) [nM] | 180 |
| Cytotoxicity (LDH release from U937 cells) (IC50) [nM] | >30000 |
| *Human FAS enzyme isolated from HeLa cells.
§Cells incubated with compound for 1 hour, 14C-acetate in Krebs–Ringer buffer incubation for 4 hours, methanol:CHCl3 1:1 extraction, measurement in ß-counter. FASN, fatty acid synthase; LDH, lactate dehydrogenase. | |
| In Vitro DMPK and CMC | |
| Probe name | BI 99179 |
| Aqueous solubility @ pH 7 [μg/ml] | >39 |
| CACO permeability @pH7.4 [*10–6 cm/s] | 94 |
| CACO efflux ratio | 0.9 |
| Microsomal stability – rat, human [% QH] | <27 |
| Plasma protein binding rat [% QH] | 97.6 |
In vivo DMPK
| In Vivo DMPK Parameters[1][2] | |
| Probe name | BI 99179 |
| t½ (h) | 3.0 |
| tmax (h) | 0.5 |
| Cmax (nM) | 2110 |
| AUC0–inf (nMh) | 9350 |
| F (%) | 46 |
| CL (ml/min/kg) | 8.2 |
| Vss (l/kg) | 1.6 |
| Cbrain,2h (nM) | 1300 |
| CCSF,2h (nM) | 50 |
| Pharmacokinetic parameters of BI 99179 in male Wistar Hannover rats.
*Fasted upon oral application of 4 mg/kg. | |
In vivo pharmacology
BI 99179 showed acute efficacy in rat models.[2]
- Increased hypothalamic Malonyl-CoA concentrations (10 or 100 mg/kg); 2 hours/24 hours post dose (p.d.)
- Decreased cumulative food intake 100 mg/kg; 24 hours p.d.
Selectivity
A screen covering 30 targets was reported: <20% inhibition at 10 μM for all targets.[1][2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Kley, Jörg T; Mack, Jürgen; Hamilton, Bradford; Scheuerer, Stefan; Redemann, Norbert (2011). "Discovery of BI 99179, a potent and selective inhibitor of type I fatty acid synthase with central exposure". Bioorganic & Medicinal Chemistry Letters. doi:10.1016/j.bmcl.2011.07.083.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 GmbH, Boehringer Ingelheim International (6 October 2017). "FAS inhibitor - BI 99179 - opnMe - Boehringer Ingelheim". https://opnme.com/molecules/fas-bi99179.
- ↑ Flavin, Richard; Peluso, Stephane; Nguyen, Paul L; Loda, Massimo (2010). "Fatty acid synthase as a potential therapeutic target in cancer". Future Oncology 6 (4): 551. doi:10.2217/fon.10.11. PMID 20373869.
- ↑ Kridel, Steven J; Lowther, W Todd; Pemble Iv, Charles W (2007). "Fatty acid synthase inhibitors: New directions for oncology". Expert Opinion on Investigational Drugs 16 (11): 1817. doi:10.1517/13543784.16.11.1817. PMID 17970640.
- ↑ Loftus, T. M; Jaworsky, D. E; Frehywot, G. L; Townsend, C. A; Ronnett, G. V; Lane, M. D; Kuhajda, F. P (2000). "Reduced Food Intake and Body Weight in Mice Treated with Fatty Acid Synthase Inhibitors". Science 288 (5475): 2379–81. doi:10.1126/science.288.5475.2379. PMID 10875926.
- ↑ Maier, T; Leibundgut, M; Ban, N (2008). "The Crystal Structure of a Mammalian Fatty Acid Synthase". Science 321 (5894): 1315. doi:10.1126/science.1161269. PMID 18772430.
Further reading
- Alexandra D'Arcangelis, John Bajor, Kimberly Campbell, Helen Knaggs. U.S. Patent 53631, 2005.
- Hardwicke, Mary Ann; Rendina, Alan R; Williams, Shawn P; Moore, Michael L; Wang, Liping; Krueger, Julie A; Plant, Ramona N; Totoritis, Rachel D et al. (2014). "A human fatty acid synthase inhibitor binds β-ketoacyl reductase in the keto-substrate site". Nature Chemical Biology 10 (9): 774. doi:10.1038/nchembio.1603. PMID 25086508.
 |