Chemistry:Santonic acid
From HandWiki
Short description: Organic compound
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(−)-2,3,3a,4,5,6,7,7a-octahydro-α,3a,5-trimethyl-6,8-dioxo-1,4-methano-1H-indene-1-acetic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C15H20O4 | |||
| Molar mass | 264.32 g mol−1 | ||
| Density | 1.184 g cm−3[1] | ||
| Melting point | 173 °C (343 °F; 446 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
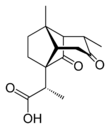
Santonic acid is an organic compound containing both carboxylic acid and ketone functionality.
It was synthesized from santonin by base-mediated hydrolysis of a lactone followed by a multistep rearrangement process by R. B. Woodward.[2]
Unusually for a carboxylic acid, santonic acid does not form hydrogen-bonded dimers in the crystalline phase. Rather, it adopts a polymeric structure, with individual santonic acid molecules linked by intermolecular carboxyl-to-ketone hydrogen bonds.[1]
References
- ↑ 1.0 1.1 1.2 A. P. J. Brunskill, H. W. Thompson and R. A. Lalancette (April 1999). "Santonic acid: catemeric hydrogen bonding in a γ,ε-diketo carboxylic acid". Acta Crystallogr. C 55 (4): 566–568. doi:10.1107/S0108270198014231.
- ↑ Reusch, William (1999). "Base-catalyzed rearrangements ". In: Virtual Textbook of Organic Chemistry. Michigan State University. Retrieved September 10, 2008.
 |