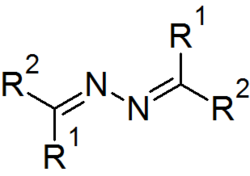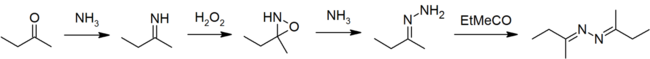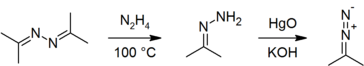Chemistry:Azine
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones.[1] For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes.[2]
Preparation
The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide.[3]
In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl.[4]
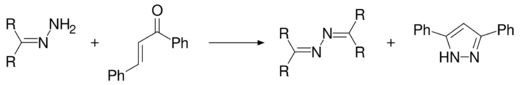
Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1H-pyrazole,[5] in a conversion also carried out with hydrazine hydrate.[6][7]
Reactions
Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone:
- R2C=N-N=CR2 + H2O → R2C=N-NH2 + R2C=O
- R2C=N-NH2 + H2O → N2H4 + R2C=O
Azines have been used as precursors to hydrazones:[4][8]
- R2C=N-N=CR2 + N2H4 → 2 R2C=N-NH2
They are also precursors to diazo compounds.[9][8][10]
The coordination chemistry of azines (as ligands) has also been studied.[11][12][13]
Acetone is used to derivatize hydrazine into acetone azine for analysis by gas chromatography. This method is used to determine trace levels of hydrazine in drinking water[14] and pharmaceuticals.[15]
Applications
Ketazines are also important intermediates in the industrial production of hydrazine hydrate by the peroxide process.[3] In the presence of an oxidant, ammonia and ketones react to give hydrazine via ketazine:
- 2 Me(Et)C=O + 2 NH3 + H2O2 → Me(Et)C=NN=C(Et)Me + 2 H2O
The ketazine can be hydrolyzed to the hydrazine and regenerate the ketone:
- Me(Et)C=NN=C(Et)Me + 2 H2O → 2 Me(Et)C=O + N2H4
Ketazines have been also used as sources of hydrazine produced in situ, for example in the production of herbicide precursor 1,2,4-triazole.[16]
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "ketazines". doi:10.1351/goldbook.K03377
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aldazines". doi:10.1351/goldbook.A00207
- ↑ 3.0 3.1 Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a13_177.
- ↑ 4.0 4.1 Day, A. C.; Whiting, M. C. (1970). "Acetone Hydrazone". Organic Syntheses 50: 3. doi:10.15227/orgsyn.050.0003.
- ↑ Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry, Section B 57B (3): 362–373. http://nopr.niscair.res.in/handle/123456789/43824.
- ↑ Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry 45 (2): 503–505. doi:10.1002/jhet.5570450231.
- ↑ Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-pot synthesis of 3,5-diphenyl-1H-pyrazoles from chalcones and hydrazine under mechanochemical ball milling". Heterocycles 89 (1): 103–112. doi:10.3987/COM-13-12867.
- ↑ 8.0 8.1 Staudinger, H.; Gaule, Alice (July 1916). "Vergleich der Stickstoff-Abspaltung bei verschiedenen aliphatischen Diazoverbindungen". Berichte der Deutschen Chemischen Gesellschaft 49 (2): 1897–1918. doi:10.1002/cber.19160490245. https://zenodo.org/record/1426609.
- ↑ Day, A. C.; Raymond, P.; Southam, R. M.; Whiting, M. C. (1966). "The preparation of secondary aliphatic diazo-compounds from hydrazones". Journal of the Chemical Society C: Organic: 467. doi:10.1039/J39660000467.
- ↑ Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. (1970). "2-Diazopropane". Organic Syntheses 50: 27. http://www.orgsyn.org/demo.aspx?prep=cv6p0392.; Collective Volume, 6, pp. 392.
- ↑ Gudkova, A. S.; Reutov, O. A.; Aleinikova, M. Ya. (August 1962). "Reactions of hydrazones and azines with metal salts". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science 11 (8): 1298–1302. doi:10.1007/BF00907973.
- ↑ Gudkova, A. S.; Aleinikova, M. Ya.; Reutov, O. A. (May 1966). "Reactions of hydrazones and azines with metal salts Communication 5. Reactions of hydrazones and azines with mercuric halides". Bulletin of the Academy of Sciences, USSR Division of Chemical Science 15 (5): 807–811. doi:10.1007/BF00849376.
- ↑ King, Fiona; Nicholls, David (January 1978). "Complex of titanium halides with acetone azine and its isomer 3, 5, 5-trimethyl-pyrazoline". Inorganica Chimica Acta 28: 55–58. doi:10.1016/S0020-1693(00)87413-7.
- ↑ Davis, II, William E.; Li, Yongtao (July 2008). "Analysis of Hydrazine in Drinking Water by Isotope Dilution Gas Chromatography/Tandem Mass Spectrometry with Derivatization and Liquid−Liquid Extraction". Analytical Chemistry 80 (14): 5449–5453. doi:10.1021/ac702536d. PMID 18564853.
- ↑ Sun, Mingjiang; Bai, Lin; Liu, David Q. (February 2009). "A generic approach for the determination of trace hydrazine in drug substances using in situ derivatization-headspace GC–MS". Journal of Pharmaceutical and Biomedical Analysis 49 (2): 529–533. doi:10.1016/j.jpba.2008.11.009. PMID 19097722.
- ↑ Nagata, Nobuhiro; Chiharu Nishizawa & Toshikiyo Kurai, "Method of producing 1,2,4-triazole", US patent 6002015, published 1999-12-14.
Further reading
- Gilbert, E. C. (1929). "Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone". J. Am. Chem. Soc. 51 (11): 3394–3409. doi:10.1021/ja01386a032.
- Eichenhofer, Kurt-Wilhelm & Reinhard Schliebs, "Production of ketazines", US patent 3965097, published 1976-06-22.
- Schirmann, Jean-Pierre; Jean Combroux & Serge Yvon Delavarenne, "Method for preparing azines and hydrazones", US patent 3972878, published 1976-08-03.
- Schirmann, Jean-Pierre; Pierre Tellier & Henri Mathais et al., "Process for the preparation of hydrazine compounds", US patent 3978049, published 1976-08-31.
- Schirmann, Jean-Pierre; Jean Combroux & Serge Yvon Delavarenne, "Method for making azines", US patent 4093656, published 1978-06-06.
- Schirmann, Jean-Pierre; Jean Combroux & Serge Y. Delavarenne, "Preparation of a concentrated aqueous solution of hydrazine hydrate", US patent 4724133, published 1988-02-09.
 |