Chemistry:Chalcone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Chalcone[2] | |
| Systematic IUPAC name
(2E)-1,3-Diphenylprop-2-en-1-one | |
| Other names
Chalkone
Benzylideneacetophenone Phenyl styryl ketone benzalacetophenone β-phenylacrylophenone γ-oxo-α,γ-diphenyl-α-propylene α-phenyl-β-benzoylethylene. | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H12O | |
| Molar mass | 208.260 g·mol−1 |
| Appearance | pale yellow solid |
| Density | 1.071 g/cm3 |
| Melting point | 55 to 57 °C (131 to 135 °F; 328 to 330 K) |
| Boiling point | 345 to 348 °C (653 to 658 °F; 618 to 621 K) |
| -125.7·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
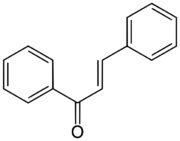
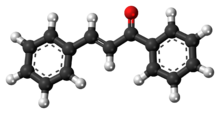
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids.[3] They are widely known bioactive substances, fluorescent materials, and chemical intermediates.
Chemical properties
Chalcones have two absorption maxima at 280 nm and 340 nm.[4]
Biosynthesis
Chalcones and chalconoids are synthesized in plants as secondary metabolites. The enzyme chalcone synthase, a type III polyketide synthase, is responsible for the biosynthesis of these compounds. The enzyme is found in all "higher" (vascular) and several "lower" (non-vascular) plants.[5]
Laboratory synthesis
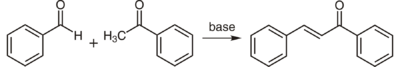
Chalcone is usually prepared by an aldol condensation between benzaldehyde and acetophenone.[6]
This reaction, which can be carried out without any solvent, is so reliable that it is often given as an example of green chemistry in undergraduate education.[7]
Potential pharmacology
Chalcones and their derivatives demonstrate a wide range of biological activities including anti-inflammation.[8] Some 2′-amino chalcones have been studied as potential antitumor agents.[9][10] Chalcones are of interest in medicinal chemistry and have been described as a privileged scaffold.[5]
Uses
Medicinal uses
In medicinal chemistry, chalcones have been used as:
- antioxidants
- Anticancer agents
- antidiabetic drugs
- antiviral drugs
- antimalarial drugs and more.
Industrial uses
In chemical industries, they are employed as:
- liquid crystals
- fluorescent chemical scaffolds
- metal sensors
- corrosion inhibitors
- plant hormones.[11]
Uses in organic chemistry
Chalcones have been used as intermediates in heterocyclic synthesis, especially in the synthesis of pyrazoles and aurones.[12]
See also
References
- ↑ Merck Index, 11th Edition, 2028
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 722. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Tomás-Barberán, Francisco A.; Clifford, Michael N. (2000). "Flavanones, Chalcones and Dihydrochalcones - Nature, Occurrence and Dietary Burden". Journal of the Science of Food and Agriculture 80 (7): 1073–1080. doi:10.1002/(SICI)1097-0010(20000515)80:7<1073::AID-JSFA568>3.0.CO;2-B.
- ↑ Song, Dong-mee; Jung, Kyoung-Hoon; Moon, Ji-hye; Shin, Dong-Myung (2003). "Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display". Optical Materials 21 (1–3): 667–71. doi:10.1016/S0925-3467(02)00220-3. Bibcode: 2003OptMa..21..667S.
- ↑ 5.0 5.1 Zhuang, Chunlin; Zhang, Wen; Sheng, Chunquan; Zhang, Wannian; Xing, Chengguo; Miao, Zhenyuan (28 June 2017). "Chalcone: A Privileged Structure in Medicinal Chemistry". Chemical Reviews 117 (12): 7762–7810. doi:10.1021/acs.chemrev.7b00020. PMID 28488435.
- ↑ E. P. Kohler, H. M. Chadwell (1922). "Benzalacetophenone". Organic Syntheses 2: 1. doi:10.15227/orgsyn.002.0001.
- ↑ Palleros, Daniel R (2004). "Solvent-Free Synthesis of Chalcones". Journal of Chemical Education 81 (9): 1345. doi:10.1021/ed081p1345. Bibcode: 2004JChEd..81.1345P.
- ↑ Mahapatra, Debarshi Kar; Bharti, Sanjay Kumar; Asati, Vivek (2017). "Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives". Current Topics in Medicinal Chemistry 17 (28): 3146–3169. doi:10.2174/1568026617666170914160446. PMID 28914193.
- ↑ Xia, Yi; Yang, Zheng-Yu; Xia, Peng; Bastow, Kenneth F.; Nakanishi, Yuka; Lee, Kuo-Hsiung (2000). "Antitumor agents. Part 202: Novel 2′-amino chalcones: design, synthesis and biological evaluation". Bioorganic & Medicinal Chemistry Letters 10 (8): 699–701. doi:10.1016/S0960-894X(00)00072-X. ISSN 0960-894X. PMID 10782667.
- ↑ Santos, Mariana B.; Pinhanelli, Vitor C.; Garcia, Mayara A.R.; Silva, Gabriel; Baek, Seung J.; França, Suzelei C.; Fachin, Ana L.; Marins, Mozart et al. (2017). "Antiproliferative and pro-apoptotic activities of 2′- and 4′-aminochalcones against tumor canine cells". European Journal of Medicinal Chemistry 138: 884–889. doi:10.1016/j.ejmech.2017.06.049. ISSN 0223-5234. PMID 28738308. https://repositorio.unesp.br/bitstream/11449/174929/1/2-s2.0-85024884865.pdf.
- ↑ Nayak, Yogeesha N.; Gaonkar, Santosh L.; Sabu, Mariya (2023-01-04). "Chalcones: Versatile intermediates in heterocyclic synthesis" (in en). Journal of Heterocyclic Chemistry: jhet.4617. doi:10.1002/jhet.4617. ISSN 0022-152X. https://onlinelibrary.wiley.com/doi/10.1002/jhet.4617.
- ↑ Nayak, Yogeesha N.; Gaonkar, Santosh L.; Sabu, Mariya (2023-01-04). "Chalcones: Versatile intermediates in heterocyclic synthesis" (in en). Journal of Heterocyclic Chemistry: jhet.4617. doi:10.1002/jhet.4617. ISSN 0022-152X. https://onlinelibrary.wiley.com/doi/10.1002/jhet.4617.
External links
 |