Chemistry:Josiphos ligands

A Josiphos ligand is a type of chiral diphosphine which has been modified to be substrate-specific; they are widely used for enantioselective synthesis.[1] They are named after the technician who made the first one, Josi Puleo.[2]
Introduction
Modern enantioselective synthesis typically applies a well-chosen homogeneous catalyst for key steps. The ligands on these catalysts are essential to a reaction's success (or failure): they influence the chemoselectivity of the catalyst, especially the catalyzed reaction's chirality. Thus a powerful technique in the development of homogenously-catalyzed reactions is to modify ligands to select the desired substrates. The Josiphos family of privileged ligands provides especially high yields in enantioselective synthesis.[3][4]
In the early 1990s, Antonio Togni began studying at the Ciba (now Novartis) Central Research Laboratories[5] previously-known[6] ferrocenyl ligands for a Au(I)-catalyzed aldol reaction.[5] Togni's team began considering diphosphine ligands, and technician Josi Puleo prepared the first ligands with secondary phosphines. The team applied Puleo's products in an Ru-catalyzed enamide hydrogenation synthesis; in a dramatic success, the reaction had e.e. >99% and a turnover frequency (TOF) 0.3 s−1.[5][6]
The same ligand proved useful in production of (S)-metolachlor, active ingredient in the most common herbicide in the United States. Synthesis requires enantioselective hydrogenation of an imine; after introduction of the catalyst, the reaction proceeds with 100% conversion, turnover number (TON) >7mil, and turnover frequency >0.5 ms−1. This process is the largest-scale application of enantioselective hydrogenation, producing over 10 kilotons/year of the desired product with 79% e.e.[1][3][7]
Josiphos ligands also serve in non-enantioselective reactions: a Pd-catalyzed reaction of aryl chlorides and aryl vinyl tosylates with TON of 20,000 or higher,[8] catalytic carbonylation,[9] or Grignard and Negishi couplings[10][11]
A variety of Josiphos ligands are commercially available under licence from Solvias. The (R-S) and its enantiomer provide higher yields and enantioselectivities than the diastereomer (R,R).[2][12]
The ferrocene scaffold has proved to be versatile.[2][13][14][15][16] One structural parameter that influences reactivity is the bite angle. The P1-M-P2 angle has an average value of 92.7°.[2]
The general consensus for the naming is abbreviating the individual ligand as (R)-(S)-R2PF-PR'2. The substituent on the Cp is written in front of the F and the R on the chiral center after the F.[1]
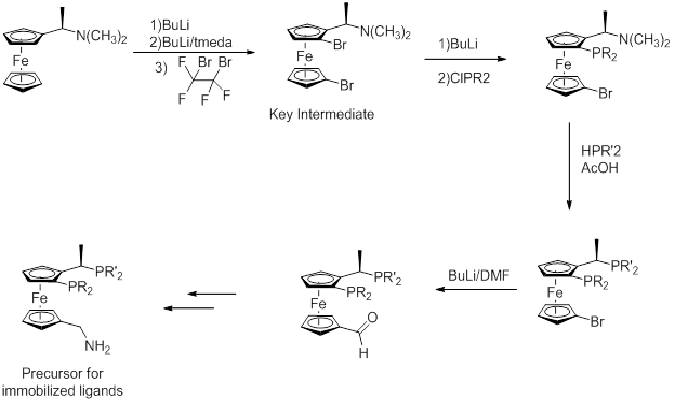
Synthesis of Josiphos ligands
The modern preparation of Josiphos ligands starts from Ugi's amine.
An important improvement on initial syntheses has been using N(CH3)2 as a leaving group over acetate, although an acetic acid solvent gives better yields.[5]
Reactions using Josiphos ligands
Some reactions that are accomplished using M-Josiphos complexes as catalyst are listed below. Other reactions where Josiphos ligands can be used are: hydrogenation of C=N, C=C and C=O bonds, catalyzed allylic substitution, hydrocarboxylation, Michael addition, allylic alkylation, Heck-type reactions, oxabicycle ring-opening, and allylamine isomerization.[citation needed]
- Hydroboration of styrene
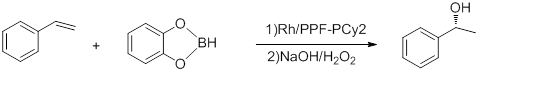
- Conducted at -78 °C, the above reaction has e.e.'s up to 92% and TOF of 5-10 h−1.[17] Hayashi's Rh-binap complex gives better yield.[18]
- Hydroformylation of Styrene
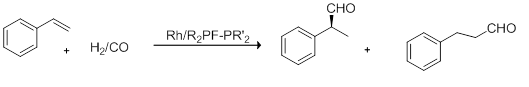
- This reaction scheme yields of up to 78% ee of the (R) product, but low TON and TOF of 10-210 and 1-14h−1 (respectively).[1][19]
- Reductive amination
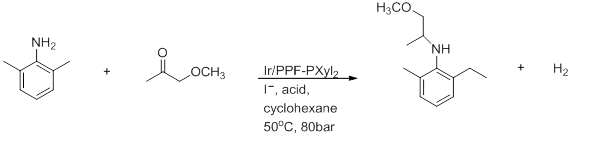
- Above is the preparation of (S)-metolachlor. Good yields and a 100% conversion crucially require AcOH solvent.[18]
- Hydrogenation of exocyclic methyl imine
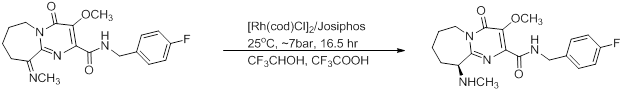
- This key step to synthesize a HIV integrase inhibitor, Crixivan, is one of the few known homogeneous heteroarene hydrogenation reactions. Bulky R groups increase the catalyst's performance, with 97% e.e. and TON and TOF of 1k and 8 min−1, respectively.[20][21]
- Asymmetric synthesis of chromanoylpyridine derivatives
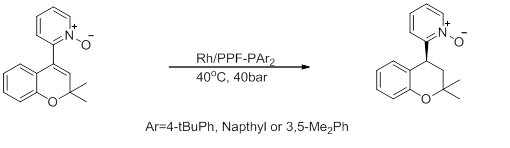
- This reaction, for an intermediate in synthesis of an antihypertensive and anti-alopecic chromanoylpyridine derivative, exhibits high enantioselectivity, but low activity.[22]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 H-U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni, Top. Catal., 2002, 19, 3.
- ↑ 2.0 2.1 2.2 2.3 Qi-Lin Zhou (2011), Privileged Chiral Ligands and Catalysts, pp. 93-127
- ↑ 3.0 3.1 Spessard, Gary and Miessler, Gary (2010). Organometallic Chemistry: Second Edition. pp. 378-379.
- ↑ Elschenbroich, Christopher (2006). Organometallics: Third Edition. pp.518-519
- ↑ 5.0 5.1 5.2 5.3 Togni, Chimia., 1996, 50, 86.
- ↑ 6.0 6.1 Ito, M. Sawamura and T. Hayashi, J. Am. Chem. Soc. 1986, 108, 6405.
- ↑ Desai, A. (4 Oct 2009). "The Story of (S)-Metolachlor: An Industrial Odyssey in Asymmetric Catalysis" (lecture slides). Lansing: Michigan State University. Archived 31 May 2017 at the Internet Archive.
- ↑ Littke, A.F and Fu, GG, Angew. Chem. Int. Ed., 2002, 41, 4176.
- ↑ Cai, C., Rivera, N.R., Balsells, J., Sidler, R.S., MC Williams, J.C., Schultz, C.S and Sun Y, Org. Lett, 2006, 8, 5161
- ↑ Limmert, M.E., Roy., A.J and Hartwig J.F, J. Org. Chem., 2005, 70, 9364
- ↑ Alvaro, E and Hartwig, J.F, J. Am. Chem. Soc., 2009, 131, 7858
- ↑ Thommen, M and Blasr, H.U Pharma Chem, 2002, 33-34
- ↑ Blaser, H.U., Malan,C., Pugin, B., Spindler, F.,Steiner, H., and Studer, M, 2003. Adv. Synth. Catal, 345, 103-152
- ↑ Whitesell, J.K Chem. Rev,. 1989, 89, 1581
- ↑ Inoguchi, K., Sakuraba, S., and Achiwa, K. Synlett, 1992, 169
- ↑ Chen, W. and Blaser, H.U 2008 in Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications. (e.d. A. Borner) pp. 359-393
- ↑ T. Hayashi, Comprehensive Asymmetric Catalyst, eds. E.N. Jacobsen, A. Pfaltz and H. Yamamoto, 1999 pp. 247
- ↑ 18.0 18.1 H.U. Blaser, H.P. Buser, H.P. Jalett, B. Pugin and F. Spindler, Synlett. 1999, 867
- ↑ Godard, C., Ruiz, A., and Claver C. Helv. Chim. Acta, 2006, 89, 1610
- ↑ R.Fuchs, EP 803502(1996) assigned to Lonza A.G
- ↑ M.Studer, C. Wedemeyer-Exl, F.Spindler and H.U Blaser, Monatsh. Chem, 2000, 131, 1335
- ↑ E. Broger, Y. Crameri and P. Jones, WO 99/01 453. (1997), assigned to Hoffman-La Roche
 |