Chemistry:Negishi coupling
| Negishi coupling | |
|---|---|
| Named after | Ei-ichi Negishi |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | negishi-coupling |
| RSC ontology ID | RXNO:0000088 |
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon–carbon bonds (C–C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used.[1][2] A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.[3][4][5]
- The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions.
- The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl.
- The halide X′ in the organozinc compound can be chloride, bromine or iodine and the organic residue R′ is alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl.
- The metal M in the catalyst is nickel or palladium
- The ligand L in the catalyst can be triphenylphosphine, dppe, BINAP, chiraphos or XPhos.
Palladium catalysts in general have higher chemical yields and higher functional group tolerance.
The Negishi coupling finds common use in the field of total synthesis as a method for selectively forming C-C bonds between complex synthetic intermediates. The reaction allows for the coupling of sp3, sp2, and sp carbon atoms, (see orbital hybridization) which make it somewhat unusual among the palladium-catalyzed coupling reactions. Organozincs are moisture and air sensitive, so the Negishi coupling must be performed in an oxygen and water free environment, a fact that has hindered its use relative to other cross-coupling reactions that require less robust conditions (i.e. Suzuki reaction). However, organozincs are more reactive than both organostannanes and organoborates which correlates to faster reaction times.
The reaction is named after Ei-ichi Negishi who was a co-recipient of the 2010 Nobel Prize in Chemistry for the discovery and development of this reaction.
Negishi and coworkers originally investigated the cross-coupling of organoaluminum reagents in 1976 initially employing Ni and Pd as the transition metal catalysts, but noted that Ni resulted in the decay of stereospecifity whereas Pd did not.[6] Transitioning from organoaluminum species to organozinc compounds Negishi and coworkers reported the use of Pd complexes in organozinc coupling reactions and carried out methods studies, eventually developing the reaction conditions into those commonly utilized today.[7] Alongside Richard F. Heck and Akira Suzuki, El-ichi Negishi was a co-recipient of the Nobel Prize in Chemistry in 2010, for his work on "palladium-catalyzed cross couplings in organic synthesis".
Reaction mechanism
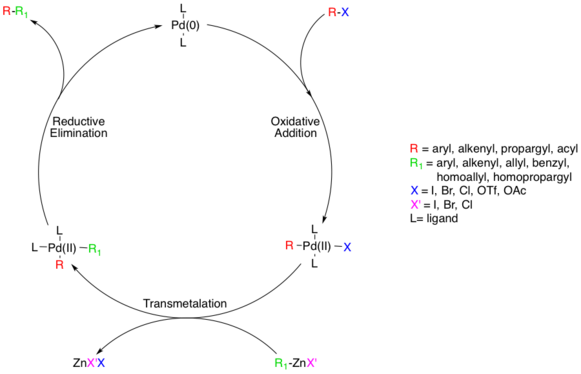
The reaction mechanism is thought to proceed via a standard Pd catalyzed cross-coupling pathway, starting with a Pd(0) species, which is oxidized to Pd(II) in an oxidative addition step involving the organohalide species.[8] This step proceeds with aryl, vinyl, alkynyl, and acyl halides, acetates, or triflates, with substrates following standard oxidative addition relative rates (I>OTf>Br≫Cl).[9]
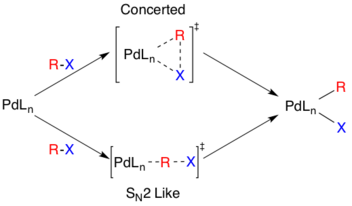
The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an SN2 like mechanism resulting in inverted stereochemistry. The other pathway proceeds via concerted addition and retains stereochemistry.
Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.[10]

Next, the transmetalation step occurs where the organozinc reagent exchanges its organic substituent with the halide in the Pd(II) complex, generating the trans- Pd(II) complex and a zinc halide salt. The organozinc substrate can be aryl, vinyl, allyl, benzyl, homoallyl, or homopropargyl.[8] Transmetalation is usually rate limiting and a complete mechanistic understanding of this step has not yet been reached though several studies have shed light on this process. Alkylzinc species form higher-order zincate species prior to transmetalation whereas arylzinc species do not.[11] ZnXR and ZnR2 can both be used as reactive reagents, and Zn is known to prefer four coordinate complexes, which means solvent coordinated Zn complexes, such as ZnXR(solvent)
2 cannot be ruled out a priori.[12] Studies indicate competing equilibriums exist between cis- and trans- bis alkyl organopalladium complexes, but that the only productive intermediate is the cis complex.[13][14]
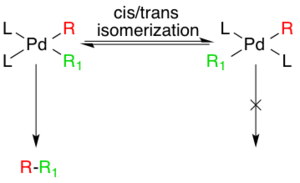
The last step in the catalytic pathway of the Negishi coupling is reductive elimination, which is thought to proceed via a three coordinate transition state, yielding the coupled organic product and regenerating the Pd(0) catalyst. For this step to occur, the aforementioned cis- alkyl organopalladium complex must be formed.[15]
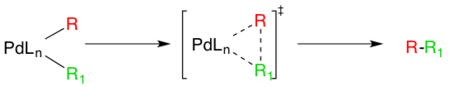
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R′ resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.[13]
A common side reaction is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide:[16]
- Ar–Pd–Ar′ + Ar′–Zn–X → Ar′–Pd–Ar′ + Ar–Zn–X
- Ar′–Pd–Ar′ → Ar′–Ar′ + Pd(0) (homocoupling)
- Ar–Zn–X + H2O → Ar–H + HO–Zn–X (reaction accompanied by dehalogenation)
Nickel catalyzed systems can operate under different mechanisms depending on the coupling partners. Unlike palladium systems which involve only Pd0 or PdII, nickel catalyzed systems can involve nickel of different oxidation states.[17] Both systems are similar in that they involve similar elementary steps: oxidative addition, transmetalation, and reductive elimination. Both systems also have to address issues of β-hydride elimination and difficult oxidative addition of alkyl electrophiles.[18]
For unactivated alkyl electrophiles, one possible mechanism is a transmetalation first mechanism. In this mechanism, the alkyl zinc species would first transmetalate with the nickel catalyst. Then the nickel would abstract the halide from the alkyl halide resulting in the alkyl radical and oxidation of nickel after addition of the radical.[19]
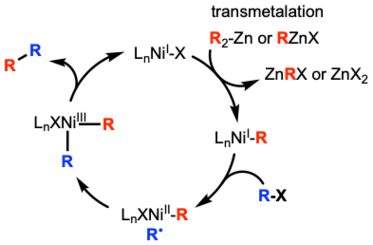
One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(o-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.[20]
Scope
The Negishi coupling has been applied the following illustrative syntheses:
- unsymmetrical 2,2'-bipyridines from 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0),[21]
- biphenyl from o-tolylzinc chloride and o-iodotoluene and tetrakis(triphenylphosphine)palladium(0),[22]
- 5,7-hexadecadiene from 1-decyne and (Z)-1-hexenyl iodide.[23]
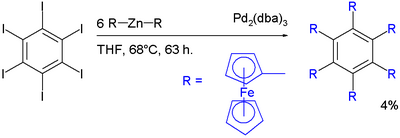
Negishi coupling has been applied in the synthesis of hexaferrocenylbenzene:[24]
with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0) in tetrahydrofuran. The yield is only 4% signifying substantial crowding around the aryl core.
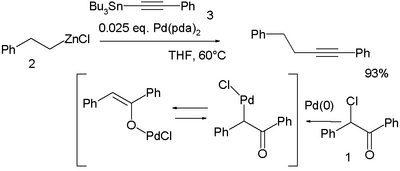
In a novel modification palladium is first oxidized by the haloketone 2-chloro-2-phenylacetophenone 1 and the resulting palladium OPdCl complex then accepts both the organozinc compound 2 and the organotin compound 3 in a double transmetalation:[25]
Examples of nickel catalyzed Negishi couplings include sp2-sp2, sp2-sp3, and sp3-sp3 systems. In the system first studied by Negishi, aryl-aryl cross coupling was catalyzed by Ni(PPh3)4 generated in situ through reduction of Ni(acac)2 with PPh3 and (i-Bu)2AlH.[26]
Variations have also been developed to allow for the cross-coupling of aryl and alkenyl partners. In the variation developed by Knochel et al, aryl zinc bromides were reacted with vinyl triflates and vinyl halides.[27]
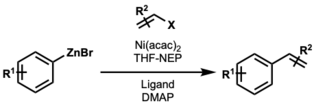
Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination.[28][29] This also works in some alkyl-aryl systems.[30]
Several asymmetric variants exist and many utilize Pybox ligands.[31][32][33]
Industrial applications
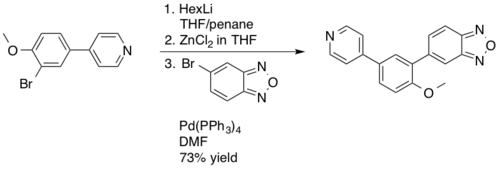
The Negishi coupling is not employed as frequently in industrial applications as its cousins the Suzuki reaction and Heck reaction, mostly as a result of the water and air sensitivity of the required aryl or alkyl zinc reagents.[34][35] In 2003 Novartis employed a Negishi coupling in the manufacture of PDE472, a phosphodiesterase type 4D inhibitor, which was being investigated as a drug lead for the treatment of asthma.[36] The Negishi coupling was used as an alternative to the Suzuki reaction providing improved yields, 73% on a 4.5 kg scale, of the desired benzodioxazole synthetic intermediate.[37]
Applications in total synthesis
Where the Negishi coupling is rarely used in industrial chemistry, a result of the aforementioned water and oxygen sensitivity, it finds wide use in the field of natural products total synthesis. The increased reactivity relative to other cross-coupling reactions makes the Negishi coupling ideal for joining complex intermediates in the synthesis of natural products.[8] Additionally, Zn is more environmentally friendly than other metals such as Sn used in the Stille coupling. The Negishi coupling historically is not used as much as the Stille or Suzuki coupling. When it comes to fragment-coupling processes the Negishi coupling is particularly useful, especially when compared to the aforementioned Stille and Suzuki coupling reactions.[38] The major drawback of the Negishi coupling, aside from its water and oxygen sensitivity, is its relative lack of functional group tolerance when compared to other cross-coupling reactions.[39]
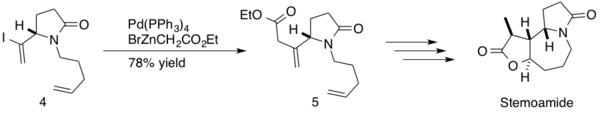
(−)-stemoamide is a natural product found in the root extracts of ‘’Stemona tuberosa’’. These extracts have been used Japanese and Chinese folk medicine to treat respiratory disorders, and (−)-stemoamide is also an anthelminthic. Somfai and coworkers employed a Negishi coupling in their synthesis of (−)-stemoamide.[40] The reaction was implemented mid-synthesis, forming an sp3-sp2 c-c bond between β,γ-unsaturated ester and an intermediate diene 4 with a 78% yield of product 5. Somfai completed the stereoselective total synthesis of (−)-stemoamide in 12-steps with a 20% overall yield.
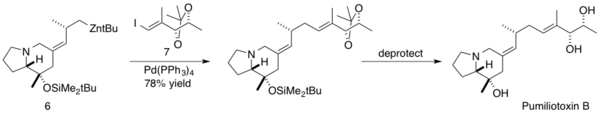
Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of Pumiliotoxin B. Pumiliotoxin B is one of the major toxic alkaloids isolated from Dendrobates pumilio, a Panamanian poison frog. These toxic alkaloids display modulatory effects on voltage-dependent sodium channels, resulting in cardiotonic and myotonic activity.[41] Kibayashi employed the Negishi coupling late stage in the synthesis of Pumiliotoxin B, coupling a homoallylic sp3 carbon on the zinc alkylidene indolizidine 6 with the (E)-vinyl iodide 7 with a 51% yield. The natural product was then obtained after deprotection.[42]
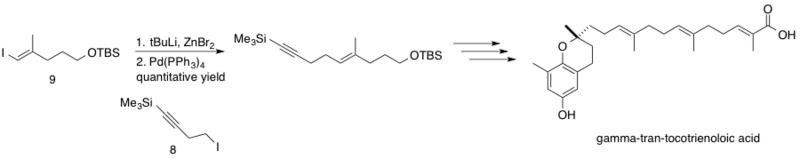
δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit DNA polymerase β (pol β), which functions to repair DNA via base excision. Inhibition of pol B in conjunction with other chemotherapy drugs may increase the cytotoxicity of these chemotherapeutics, leading to lower effective dosages. The Negishi coupling was implemented in the synthesis of δ-trans-tocotrienoloic acid by Hecht and Maloney coupling the sp3 homopropargyl zinc reagent 8 with sp2 vinyl iodide 9.[43] The reaction proceeded with quantitative yield, coupling fragments mid-synthesis en route to the stereoselectively synthesized natural product δ-trans-tocotrienoloic acid.
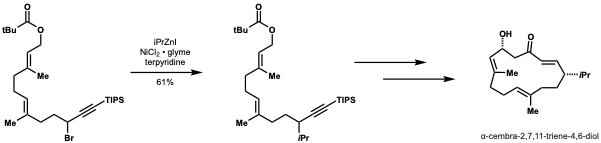
Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an iso-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.[44]
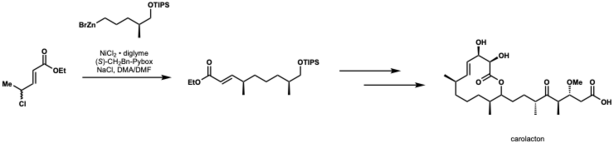
Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolacton. In this application, they achieved 82% yield and dr = 10:1.[45]
Preparation of organozinc precursors
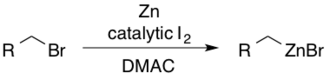
Alkylzinc reagents can be accessed from the corresponding alkyl bromides using iodine in dimethylacetamide (DMAC).[46] The catalytic I2 serves to activate the zinc towards nucleophilic addition.
Aryl zincs can be synthesized using mild reaction conditions via a Grignard like intermediate.[47]
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.[48]
Further reading
- "Higher-order zincates as transmetalators in alkyl-alkyl negishi cross-coupling". Angewandte Chemie 51 (28): 7024–7. July 2012. doi:10.1002/anie.201203547. PMID 22685029.
- "Mild and general conditions for negishi cross-coupling enabled by the use of palladacycle precatalysts". Angewandte Chemie 52 (2): 615–9. January 2013. doi:10.1002/anie.201207750. PMID 23172689.
See also
- CPhos
- Heck reaction
- Suzuki reaction
References
- ↑ King, Anthony O.; Okukado, Nobuhisa; Negishi, Ei-ichi (1977). "Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides". Journal of the Chemical Society, Chemical Communications (19): 683. doi:10.1039/C39770000683.
- ↑ Kürti, László; Czakó, Barbara (2007). Strategic applications of named reactions in organic synthesis : background and detailed mechanisms ; 250 named reactions. Amsterdam: Elsevier Academic Press. ISBN 978-0-12-429785-2.
- ↑ Zhou, Jianrong (Steve); Fu, Gregory C. (December 2003). "Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides" (in en). Journal of the American Chemical Society 125 (48): 14726–14727. doi:10.1021/ja0389366. ISSN 0002-7863. PMID 14640646. Bibcode: 2003JAChS.12514726Z. https://pubs.acs.org/doi/10.1021/ja0389366.
- ↑ Negishi, Eiichi; King, Anthony O.; Okukado, Nobuhisa (1977-05-01). "Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides". The Journal of Organic Chemistry 42 (10): 1821–1823. doi:10.1021/jo00430a041. ISSN 0022-3263. https://doi.org/10.1021/jo00430a041.
- ↑ Gavryushin, Andrei; Kofink, Christiane; Manolikakes, Georg; Knochel, Paul (2005-10-01). "Efficient Cross-Coupling of Functionalized Arylzinc Halides Catalyzed by a Nickel Chloride−Diethyl Phosphite System". Organic Letters 7 (22): 4871–4874. doi:10.1021/ol051615+. ISSN 1523-7060. PMID 16235910. https://doi.org/10.1021/ol051615+.
- ↑ "A novel stereospecific alkenyl-alkenyl cross-coupling by a palladium- or nickel-catalyzed reaction of alkenylalanes with alkenyl halides". Journal of the American Chemical Society 98 (21): 6729–6731. 1976. doi:10.1021/ja00437a067. Bibcode: 1976JAChS..98.6729B.
- ↑ "Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides". The Journal of Organic Chemistry 42 (10): 1821–1823. 1977. doi:10.1021/jo00430a041.
- ↑ 8.0 8.1 8.2 Strategic Applications of Named Reactions in Organic Synthesis. New York: Elsevier Academic Press. 2005.
- ↑ Andrew G Myers Research Group. "Chemistry 115 Handouts". Boston, Massachusetts: Harvard University Department of Chemistry. https://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts.
- ↑ "On the Configuration Resulting from Oxidative Addition of RX to Pd(PPh3)4 and the Mechanism of the cis-to-trans Isomerization of [PdRX(PPh3)2] Complexes (R = Aryl, X = Halide)". Organometallics 17 (5): 954–959. 1998. doi:10.1021/om9709502.
- ↑ "Higher-order zincates as transmetalators in alkyl-alkyl negishi cross-coupling". Angewandte Chemie 51 (28): 7024–7. July 2012. doi:10.1002/anie.201203547. PMID 22685029.
- ↑ "Computational perspective on Pd-catalyzed C-C cross-coupling reaction mechanisms". Accounts of Chemical Research 46 (11): 2626–34. November 2013. doi:10.1021/ar400080r. PMID 23848308.
- ↑ 13.0 13.1 "Insights into the mechanism of the Negishi reaction: ZnRX versus ZnR2 reagents". Journal of the American Chemical Society 129 (12): 3508–9. March 2007. doi:10.1021/ja070235b. PMID 17328551. Bibcode: 2007JAChS.129.3508C.
- ↑ "Palladium round trip in the Negishi coupling of trans-[PdMeCl(PMePh2)2] with ZnMeCl: an experimental and DFT study of the transmetalation step". Chemistry: A European Journal 16 (29): 8596–9. August 2010. doi:10.1002/chem.201001332. PMID 20623568.
- ↑ The Organometallic Chemistry of the Transition Metals. 4. Hoboken, NJ: John Wiley and Sons Inc.. 2005.
- ↑ "Revealing a second transmetalation step in the Negishi coupling and its competition with reductive elimination: improvement in the interpretation of the mechanism of biaryl syntheses". Journal of the American Chemical Society 131 (29): 10201–10. July 2009. doi:10.1021/ja903277d. PMID 19572717. Bibcode: 2009JAChS.13110201L.
- ↑ Phapale, Vilas B.; Cárdenas, Diego J. (2009-05-27). "Nickel-catalysed Negishi cross-coupling reactions: scope and mechanisms" (in en). Chemical Society Reviews 38 (6): 1598–1607. doi:10.1039/B805648J. ISSN 1460-4744. PMID 19587955. https://pubs.rsc.org/en/content/articlelanding/2009/cs/b805648j.
- ↑ Zhou, Jianrong (Steve); Fu, Gregory C. (December 2003). "Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides" (in en). Journal of the American Chemical Society 125 (48): 14726–14727. doi:10.1021/ja0389366. ISSN 0002-7863. PMID 14640646. Bibcode: 2003JAChS.12514726Z. https://pubs.acs.org/doi/10.1021/ja0389366.
- ↑ Schley, Nathan D.; Fu, Gregory C. (2014-11-26). "Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation". Journal of the American Chemical Society 136 (47): 16588–16593. doi:10.1021/ja508718m. ISSN 0002-7863. PMID 25402209. PMC 4277758. Bibcode: 2014JAChS.13616588S. https://doi.org/10.1021/ja508718m.
- ↑ Morrell, Dennis G.; Kochi, Jay K. (1975-12-01). "Mechanistic studies of nickel catalysis in the cross coupling of aryl halides with alkylmetals. Role of arylalkylnickel(II) species as intermediates". Journal of the American Chemical Society 97 (25): 7262–7270. doi:10.1021/ja00858a011. ISSN 0002-7863. Bibcode: 1975JAChS..97.7262M. https://doi.org/10.1021/ja00858a011.
- ↑ Adam P. Smith, Scott A. Savage, J. Christopher Love, and Cassandra L. Fraser (2004). "Synthesis of 4-, 5-, and 6-methyl-2,2'-bipyridine by a Negishi cross-coupling strategy: 5-methyl-2,2'-bipyridine". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=v78p0051.; Collective Volume, 10, pp. 517
- ↑ Ei-ichi Negishi, Tamotsu Takahashi, and Anthony O. King (1993). "Synthesis of biaryls via palladium-catalyzed cross-coupling: 2-methyl-4'-nitrobiphenyl". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv8p0430.; Collective Volume, 8, pp. 430
- ↑ Ei-ichi Negishi, Tamotsu Takahashi, and Shigeru Baba (1993). "Palladium-catalyzed synthesis of conjugated dienes". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv8p0295.; Collective Volume, 8, pp. 295
- ↑ "Hexaferrocenylbenzene". Chemical Communications (24): 2572–4. June 2006. doi:10.1039/b604844g. PMID 16779481. https://zenodo.org/record/896672.
- ↑ "Oxidative cross-coupling through double transmetallation: surprisingly high selectivity for palladium-catalyzed cross-coupling of alkylzinc and alkynylstannanes". Journal of the American Chemical Society 128 (47): 15048–9. November 2006. doi:10.1021/ja0647351. PMID 17117830. Bibcode: 2006JAChS.12815048Z.
- ↑ Negishi, Eiichi; King, Anthony O.; Okukado, Nobuhisa (1977-05-01). "Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides". The Journal of Organic Chemistry 42 (10): 1821–1823. doi:10.1021/jo00430a041. ISSN 0022-3263. https://doi.org/10.1021/jo00430a041.
- ↑ Gavryushin, Andrei; Kofink, Christiane; Manolikakes, Georg; Knochel, Paul (2005-10-01). "Efficient Cross-Coupling of Functionalized Arylzinc Halides Catalyzed by a Nickel Chloride−Diethyl Phosphite System". Organic Letters 7 (22): 4871–4874. doi:10.1021/ol051615+. ISSN 1523-7060. PMID 16235910. https://doi.org/10.1021/ol051615+.
- ↑ Giovannini, Riccardo; Stüdemann, Thomas; Dussin, Gaelle; Knochel, Paul (1998). "An Efficient Nickel-Catalyzed Cross-Coupling Between sp3 Carbon Centers" (in en). Angewandte Chemie International Edition 37 (17): 2387–2390. doi:10.1002/(SICI)1521-3773(19980918)37:17<2387::AID-ANIE2387>3.0.CO;2-M. ISSN 1521-3773. PMID 29710957. https://onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291521-3773%2819980918%2937%3A17%3C2387%3A%3AAID-ANIE2387%3E3.0.CO%3B2-M.
- ↑ Jensen, Anne Eeg; Knochel, Paul (2002-01-01). "Nickel-Catalyzed Cross-Coupling between Functionalized Primary or Secondary Alkylzinc Halides and Primary Alkyl Halides". The Journal of Organic Chemistry 67 (1): 79–85. doi:10.1021/jo0105787. ISSN 0022-3263. PMID 11777442. https://doi.org/10.1021/jo0105787.
- ↑ Giovannini, Riccardo; Knochel, Paul (1998-11-01). "Ni(II)-Catalyzed Cross-Coupling between Polyfunctional Arylzinc Derivatives and Primary Alkyl Iodides". Journal of the American Chemical Society 120 (43): 11186–11187. doi:10.1021/ja982520o. ISSN 0002-7863. Bibcode: 1998JAChS.12011186G. https://doi.org/10.1021/ja982520o.
- ↑ Fischer, Christian; Fu, Gregory C. (April 2005). "Asymmetric Nickel-Catalyzed Negishi Cross-Couplings of Secondary α-Bromo Amides with Organozinc Reagents" (in en). Journal of the American Chemical Society 127 (13): 4594–4595. doi:10.1021/ja0506509. ISSN 0002-7863. PMID 15796523. Bibcode: 2005JAChS.127.4594F. https://pubs.acs.org/doi/10.1021/ja0506509.
- ↑ Son, Sunghee; Fu, Gregory C. (2008-03-01). "Nickel-Catalyzed Asymmetric Negishi Cross-Couplings of Secondary Allylic Chlorides with Alkylzincs" (in en). Journal of the American Chemical Society 130 (9): 2756–2757. doi:10.1021/ja800103z. ISSN 0002-7863. PMID 18257579. Bibcode: 2008JAChS.130.2756S. https://pubs.acs.org/doi/10.1021/ja800103z.
- ↑ Phapale, Vilas B.; Buñuel, Elena; García-Iglesias, Miguel; Cárdenas, Diego J. (2007). "Ni-Catalyzed Cascade Formation of C(sp3)C(sp3) Bonds by Cyclization and Cross-Coupling Reactions of Iodoalkanes with Alkyl Zinc Halides". Angewandte Chemie International Edition 46 (46): 8790–8795. doi:10.1002/anie.200702528. ISSN 1521-3773. PMID 17918274. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.200702528.
- ↑ "Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize". Angewandte Chemie 51 (21): 5062–85. May 2012. doi:10.1002/anie.201107017. PMID 22573393. https://durham-repository.worktribe.com/output/1323930.
- ↑ "One-pot Negishi cross-coupling reactions of in situ generated zinc reagents with aryl chlorides, bromides, and triflates". The Journal of Organic Chemistry 73 (18): 7380–2. September 2008. doi:10.1021/jo801063c. PMID 18693766.
- ↑ "Large-Scale Negishi Coupling as Applied to the Synthesis of PDE472, an Inhibitor of Phosphodiesterase Type 4D". Organic Process Research & Development 7 (3): 436–445. 2003. doi:10.1021/op025615q.
- ↑ "Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries". Advanced Synthesis & Catalysis 351 (18): 3027–3043. 2009. doi:10.1002/adsc.200900587.
- ↑ "Palladium-catalyzed cross-coupling reactions in total synthesis". Angewandte Chemie 44 (29): 4442–89. July 2005. doi:10.1002/anie.200500368. PMID 15991198.
- ↑ "Advances in the Negishi coupling". Aust. J. Chem. 57 (1): 107. 2004. doi:10.1071/ch03225.
- ↑ "Total synthesis of (-)-stemoamide". The Journal of Organic Chemistry 72 (11): 4246–9. May 2007. doi:10.1021/jo070498o. PMID 17451274.
- ↑ "Interaction of pumiliotoxin B with an "alkaloid-binding domain" on the voltage-dependent sodium channel". Molecular Pharmacology 42 (6): 1104–8. December 1992. doi:10.1016/S0026-895X(25)09259-4. PMID 1336116.
- ↑ "Convergent Approach to Pumiliotoxin Alkaloids. Asymmetric Total Synthesis of (+)-Pumiliotoxins A, B, and 225F". The Journal of Organic Chemistry 67 (16): 5517–5526. 2002. doi:10.1021/jo0200466. PMID 12153249.
- ↑ "A stereocontrolled synthesis of delta-trans-tocotrienoloic acid". Organic Letters 7 (19): 4297–300. September 2005. doi:10.1021/ol051849t. PMID 16146411.
- ↑ Smith, Sean W.; Fu, Gregory C. (2008). "Nickel-Catalyzed Negishi Cross-Couplings of Secondary Nucleophiles with Secondary Propargylic Electrophiles at Room Temperature". Angewandte Chemie International Edition 47 (48): 9334–9336. doi:10.1002/anie.200802784. ISSN 1521-3773. PMID 18972493.
- ↑ Schmidt, Thomas; Kirschning, Andreas (2012). "Total Synthesis of Carolacton, a Highly Potent Biofilm Inhibitor". Angewandte Chemie International Edition 51 (4): 1063–1066. doi:10.1002/anie.201106762. ISSN 1521-3773. PMID 22162345. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201106762.
- ↑ "Highly efficient, general procedure for the preparation of alkylzinc reagents from unactivated alkyl bromides and chlorides". Organic Letters 5 (4): 423–5. February 2003. doi:10.1021/ol0272693. PMID 12583734.
- ↑ "Ni(II)-Catalyzed Cross-Coupling between Polyfunctional Arylzinc Derivatives and Primary Alkyl Iodides". Journal of the American Chemical Society 120 (43): 11186–11187. 1998. doi:10.1021/ja982520o. Bibcode: 1998JAChS.12011186G.
- ↑ Sase, Shohei; Jaric, Milica; Metzger, Albrecht; Malakhov, Vladimir; Knochel, Paul (2008-09-19). "One-Pot Negishi Cross-Coupling Reactions of In Situ Generated Zinc Reagents with Aryl Chlorides, Bromides, and Triflates" (in en). The Journal of Organic Chemistry 73 (18): 7380–7382. doi:10.1021/jo801063c. ISSN 0022-3263. PMID 18693766. https://pubs.acs.org/doi/10.1021/jo801063c.
External links
- The Negishi coupling at www.organic-chemistry.org
 |