Chemistry:Indole-5,6-quinone
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1H-Indole-5,6-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
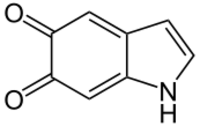
| C8H5NO2 | |
| Molar mass | 147.13 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Indole-5,6-quinone is an indolequinone, a chemical compound found in the oxidative browning reaction of fruits like bananas where it is mediated by the tyrosinase type polyphenol oxidase from tyrosine and catecholamines leading to the formation of catechol melanin.[1] Like many quinones it can undergo redox reactions via the corresponding 5,6-dihydroxyindole.[2]
See also
References
- ↑ Molecular Basis of Catecholamine Biosynthesis in Banana Fruit. Thesis submitted to the R.H. Smith Faculty of Agriculture, Food and Environment Quality Sciences of the Hebrew University of Jerusalem for the degree of Master of Science in Agriculture by Lydia Quansah, March 2009
- ↑ Beer, R. J. S.; Broadhurst, Tom; Robertson, Alexander (1954). "The chemistry of the melanins. Part V. The autoxidation of 5 : 6-dihydroxyindoles". Journal of the Chemical Society (Resumed): 1947. doi:10.1039/JR9540001947.
 |

