Chemistry:Rosaramicin
From HandWiki
| |
| Names | |
|---|---|
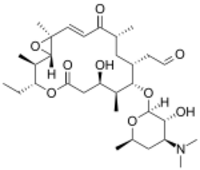
| IUPAC name
{(1S,2R,3R,7R,8S,9S,10R,12R,14E,16S)-3-Ethyl-7-hydroxy-2,8,12,16-tetramethyl-5,13-dioxo-9-[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyloxy]-4,17-dioxabicyclo[14.1.0]heptadec-14-en-10-yl}acetaldehyde
| |
| Systematic IUPAC name
[(1S,2R,3R,7R,8S,9S,10R,12R,14E,16S)-9-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-3-ethyl-7-hydroxy-2,8,12,16-tetramethyl-5,13-dioxo-4,17-dioxabicyclo[14.1.0]heptadec-14-en-10-yl]acetaldehyde | |
| Other names
Rosamicin; Juvenimicin A3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C31H51NO9 | |
| Molar mass | 581.747 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Rosaramicin (rosamicin) is an antibacterial substance that is chemically a lipid-soluble basic macrolide similar to erythromycin but with a better activity against Gram-negative bacteria.[citation needed]
Experiments in dogs have shown that it is more concentrated in the prostate than erythromycin is, and thus may be better for treating infections of that organ.[1]
References
 |

