Chemistry:Oxepanoprolinamide
From HandWiki
Short description: Class of chemical compounds
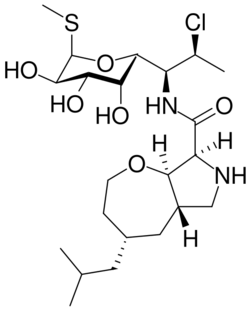
Oxepanoprolinamides are a class of antibiotics. They include iboxamycin.[1] These drugs are fully synthetic. The molecules contain the aminooctose component of clindamycin. They were developed by Andrew G. Myers and Yury S. Polikanov.[1] The structure contains an oxepane (a seven-membered ring compound with oxygen) and a proline, with an amide group that increases rigidity.[2]
Oxepanoprolinamides function by insertion into bacterial ribosomes. They overcome a type of antibiotic resistance to clindamycin based on Erm and Cfr ribosomal RNA methyltransferase enzymes.[1][3][4]
References
- ↑ 1.0 1.1 1.2 Halford, Bethany (28 October 2021). "New class of synthetic antibiotics battle drug-resistant bacteria". Chemical & Engineering News 99 (40). https://cen.acs.org/pharmaceuticals/drug-discovery/New-class-synthetic-antibiotics-battle/99/i40.
- ↑ Mitcheltree, Matthew J.; Pisipati, Amarnath; Syroegin, Egor A.; Silvestre, Katherine J.; Klepacki, Dorota; Mason, Jeremy D.; Terwilliger, Daniel W.; Testolin, Giambattista et al. (2021). "A synthetic antibiotic class overcoming bacterial multidrug resistance". Nature 599 (7885): 507–599. doi:10.1038/s41586-021-04045-6. PMID 34707295. Bibcode: 2021Natur.599..507M.
- ↑ Mitcheltree, Matthew J.; Pisipati, Amarnath; Syroegin, Egor A.; Silvestre, Katherine J.; Klepacki, Dorota; Mason, Jeremy D.; Terwilliger, Daniel W.; Testolin, Giambattista et al. (27 October 2021). "A synthetic antibiotic class overcoming bacterial multidrug resistance". Nature 599 (7885): 507–512. doi:10.1038/s41586-021-04045-6. PMID 34707295. Bibcode: 2021Natur.599..507M.
- ↑ "Bacterial drug resistance overcome by synthetic restructuring of antibiotics". Nature: d41586–021–02916-6. 27 October 2021. doi:10.1038/d41586-021-02916-6. PMID 34711985.
 |