Chemistry:Allyl hexanoate
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-en-1-yl hexanoate | |
| Other names
Prop-2-enyl hexanoate
Allyl hexanoate Allyl caproate Allyl n-caproate 2-Propenyl n-hexanoate Hexanoic acid 2-propenyl ester | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H16O2 | |
| Molar mass | 156.225 g·mol−1 |
| Appearance | Colorless to pale yellow clear liquid[1] |
| Density | 0.887 g/mL[2] 0.884-0.892 g/mL[1] |
| Boiling point | 190 to 191 °C (374 to 376 °F; 463 to 464 K)[1] 75-76 °C (15 mmHg)[2] |
| Insoluble[1] | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H311, H315, H319, H331, H410, H411, H412 | |
| P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P305+351+338, P311, P312, P321, P322, P330, P332+313, P337+313, P361, P362, P363, P391, P403+233, P405, P501 | |
| Flash point | 66 °C (151 °F; 339 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
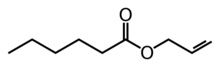
Allyl hexanoate is an organic compound with the formula C5H11CO2CH2CH=CH2. It is a colorless liquid, although commercial samples appear yellowish. It occurs naturally in pineapples.[3]
Uses
Allyl hexanoate is employed principally in the formulation of pineapple flavors but it can also be used for peach and apricot essences and for apple blossom, peach blossom, and wisteria perfume compositions.[1] It is an ingredient of some lipstick perfumes and cigarette tobacco. It also adds a sweet juicy note to citrus flavors.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Allyl hexanoate at The Good Scents Company
- ↑ 2.0 2.1 Allyl caproate at Sigma-Aldrich
- ↑ Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim.doi:10.1002/14356007.t11_t01
 |

