Chemistry:Velleral
From HandWiki
| |
| Names | |
|---|---|
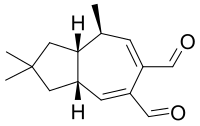
| Preferred IUPAC name
(3aR,8R,8aR)-2,2,8-Trimethyl-1,2,3,3a,8,8a-hexahydroazulene-5,6-dicarbaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H20O2 | |
| Molar mass | 232.323 g·mol−1 |
| Density | 1.093 g/cm3 |
| Hazards | |
| Flash point | 127.95 °C (262.31 °F; 401.10 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Velleral (2,2,8-trimethyl-3,3a,8,8a-tetrahydro-1H-azulene-5,6-dicarbaldehyde) is a sesquiterpene dialdehyde found in certain mushrooms, like Lactarius torminosus[1] and Lactarius vellereus, after which it was named. The compound is thought to be part of a chemical defense system that protects the mushrooms against predation.[2] First isolated in 1969,[3] and characterized structurally in 1973,[4] velleral has antimicrobial activity.[5] Several syntheses have been devised.[6][7][8]
References
- ↑ "Labile toxic compounds of the Lactarii: the role of the laticiferous hyphae as a storage depot for precursors of pungent dialdehydes". Mycologia 76 (2): 355–8. 1984. doi:10.2307/3793113. http://www.cybertruffle.org.uk/cyberliber/59350/0076/002/0355.htm.
- ↑ "The sesquiterpenes of Lactarius vellereus and their role in a proposed chemical defense system". Journal of Natural Products 48 (2): 279–88. 1985. doi:10.1021/np50038a013.
- ↑ "Velleral und iso-Velleral, scharf schmeckende Stoffe aus Lactarius vellereus FRIES" (in German). Archiv der Pharmazie 302 (2): 125–43. 1969. doi:10.1002/ardp.19693020208. PMID 5260842.
- ↑ "Fungal extractives .4. Structure of a novel sesquiterpene dialdehyde from Lactarius by spectroscopic methods". Tetrahedron 29 (11): 1621–4. 1973. doi:10.1016/S0040-4020(01)83407-4.
- ↑ "Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms". Planta Medica 57 (4): 344–6. 1991. doi:10.1055/s-2006-960114. PMID 1775575.
- ↑ "Fungal extractives .9. Synthesis of velleral skeleton and a total synthesis of pyrovellerolactone". Journal of Organic Chemistry 40 (11): 1595–601. 1975. doi:10.1021/jo00899a017.
- ↑ "Fungal extractives .10. Alternative synthesis of velleral skeleton". Journal of Organic Chemistry 41 (22): 3518–20. 1976. doi:10.1021/jo00884a005.
- ↑ "Fungal extractives .12. Construction of vellerane skeleton with total syntheses of racemic velleral, vellerolactone, and pyrovellerolactone – revised structures". Journal of the American Chemical Society 100 (21): 6728–33. 1978. doi:10.1021/ja00489a030.
 |

