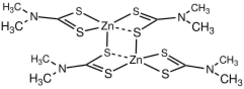
Chemistry:Zinc bis(dimethyldithiocarbamate)
| |
| Names | |
|---|---|
| IUPAC name
(μ-Dimethylcarbamodithioato-1κS,2κS′)(μ-dimethylcarbamodithioato-1κS′,2κS)bis[(dimethyl
| |
| Other names
zinc dimethyldithiocarbamate, Ziram
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12N2S4Zn | |
| Molar mass | 305.80 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.[1]
Applications
Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent.[2] As a protectant fungicide, it is active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be directly sprayed on to a plant’s leaf or it can be used as a soil and seed treatment. The top five crops ziram is used on are: almonds, peaches, nectarines, pears, and table and raisin grapes.[3]
Alternatively, ziram is used as an additive ingredient in industrial adhesives, caulking, and paint. It also serves as a bird and mammal repellent on outdoor ornamental items.
Chemistry
The compound is a prototypical zinc dithiocarbamate, a broad class of coordination complexes with the formulae Zn(R2NCS2)2, where R can be varied. Such compounds are produced by treating zinc and dithiocarbamate (R2NCS2−), as illustrated with dimethyldithiocarbamate:[4]
- 2 (CH3)2NCS2− + Zn2+ → Zn((CH3)2NCS2)2
Annually, approximately 1.9 million pounds of the active ziram ingredient are used. Ziram is often sold in powder or granule form.[2]
Zinc bis(diethyldithiocarbamate) complexes degrade thermally to give zinc sulfide.[5]
Structure
Compounds of the type Zn(S2CNR2)2 are dimeric, i.e. their proper formula is [Zn(S2CNR2)2]2.[6] Each Zn center is in a distorted pentacoordinate site, with four Zn-S bonds of 2.3 Å length and one Zn---S interaction >2.8 Å in length. Mono-zinc derivatives are obtained by adding strong ligands (L) such as amines, which give adducts Zn(S2CNR2)2L.[7]
Ecological effects
The U.S. Environmental Protection Agency has concluded that ziram poses a low toxicity risk to mammals, a moderate risk to birds, and a high risk to aquatic species. After reviewing studies that investigated the effect of ziram on aquatic organisms, the Pesticide Action Network Pesticide Database concluded that its LC50 dose (amount of pesticide that is lethal to 50% of the test organisms within the stated study time) for amphibians places it in the "highly toxic" category.
See also
- Iron tris(dimethyldithiocarbamate) - a related complex, but with three dimethyldithiocarbamate ligands
- Nickel bis(dimethyldithiocarbamate) - a related complex, but where zinc has been replaced with nickel
- Zinc bis(diethyldithiocarbamate) - a closely related complex, but where methyl has been replaced with ethyl
References
- ↑ Van Gysel, August B.; Musin, Willy (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_535.
- ↑ 2.0 2.1 "Ziram". United States Environmental Protection Agency. http://www.epa.gov/opp00001/reregistration/REDs/factsheets/ziram_red_fs.pdf.
- ↑ "Ziram". Cornell University, Oregon State University, the University of Idaho, and the University of California at Davis and the Institute for Environmental Toxicology, Michigan State University. http://extoxnet.orst.edu/pips/ziram.htm.
- ↑ Rüdiger Schubart (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001. ISBN 3527306730.
- ↑ Shen, Shuling; Zhang, Yejun; Peng, Long; Xu, Bing; Du, Yaping; Deng, Manjiao; Xu, Huarui; Wang, Qiangbin (2011). "Generalized Synthesis of Metal Sulfide Nanocrystals from Single-Source Precursors: Size, Shape and Chemical Composition Control and Their Properties". CrystEngComm 13 (14): 4572. doi:10.1039/c0ce00982b. ISSN 1466-8033.
- ↑ 6.0 6.1 Mahid Motevalli; PaulO'Brien; John R.Walsh; Ian M.Watson (1996). "Synthesis, characterization and x-ray crystal structures of asymmetric bis(dialkyldithiocarbamates) of zinc: Potential precursors for ZnS deposition". Polyhedron 15 (16): 2801–2808. doi:10.1016/0277-5387(95)00559-5.
- ↑ N. Sreehari; Babu Varghese; P. T. Manoharan (1990). "Crystal and molecular structure of dimeric bis[N,N-di-n-propyldithiocarbamato]zinc(II) and the study of exchange-coupled copper(II)-copper(II) pairs in its lattice". Inorg. Chem. 29 (20): 4011–4015. doi:10.1021/ic00345a020.
External links
- http://pmep.cce.cornell.edu/profiles/extoxnet/pyrethrins-ziram/ziram-ext.html
- Zinc bis(dimethyldithiocarbamate) in the Pesticide Properties DataBase (PPDB)
 |

