Biology:Dimethyldithiocarbamate
From HandWiki
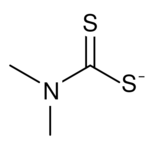
Dimethyldithiocarbamate is the organosulfur anion with the formula (CH3)2NCS2−. It is one of the simplest organic dithiocarbamate.
Uses
It is a component of various pesticides and rubber chemicals in the form of its salts sodium dimethyldithiocarbamate, and potassium dimethyldithiocarbamate) as well as its complexes zinc dimethyldithiocarbamate, ferric dimethyldithiocarbamate, and nickel bis(dimethyldithiocarbamate). Oxidation gives thiram.[1][2] thumb|right|222px|[[Iron tris(dimethyldithiocarbamate) (Fe(S2CNMe2)3) is illustrative of hundreds of known dithiocarbamate complexes.[3]]]
References
- ↑ "Dimethyldithiocarbamate salts". Environmental Protection Agency. https://archive.epa.gov/pesticides/reregistration/web/html/index-84.html.
- ↑ Rüdiger Schubart (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001. ISBN 3527306730.
- ↑ D. Coucouvanis (2007). "The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes". Progress in Inorganic Chemistry 11: 233–371. doi:10.1002/9780470166123.ch4. ISBN 9780470166123.
 |