Chemistry:2-Nitronaphthalene
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| 2046354 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2538 |
| |
| |
| Properties | |
| C10H7NO2 | |
| Molar mass | 173.171 g·mol−1 |
| Appearance | colorless solid |
| Density | 1,31 g·cm−3 |
| Melting point | 79 °C (174 °F; 352 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H350, H411 | |
| P203Script error: No such module "Preview warning".Category:GHS errors, P273, P280, P318Script error: No such module "Preview warning".Category:GHS errors, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
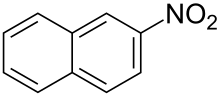
2-Nitronaphthalene is an organic compound with the formula C
10H
7NO
2. It is one of two isomers of nitronaphthalene, the other being 1-nitronaphthalene. 2-Nitronaphthalene is produced in very low yields upon nitration of naphthalene, but it can be more efficiently obtained via the diazotization of 2-aminonaphthalene.[2]
References
- ↑ "2-Nitronaphthalene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11392#section=Safety-and-Hazards.
- ↑ Booth, Gerald (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
 |

