Chemistry:Triisopropylphosphine
From HandWiki
Short description: Chemical compound
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tri(propan-2-yl)phosphane | |||
| Other names
Triisopropylphosphine
PiPr3 Pi-Pr3 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C9H21P | |||
| Molar mass | 160.24 g mol−1 | ||
| Appearance | colourless liquid | ||
| Density | 0.839 g/mL | ||
| Boiling point | 81 °C (178 °F; 354 K) (22 mm Hg) | ||
| good in alkanes | |||
| Hazards | |||
| Main hazards | spontaneously flammable | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
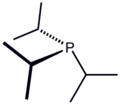
Triisopropylphosphine is the tertiary phosphine with the formula P(CH(CH3)2)3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to Pi-Pr3 or PiPr3. This ligand is one of the most basic alkyl phosphines with a large ligand cone angle of 160.[1]
Pi-Pr3 is similar to the more frequently used tricyclohexylphosphine. The triisopropyl derivative however, is a liquid at room temperature and more soluble in hydrocarbons.
References
- ↑ C. A. Tolman (1977). "Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis". Chem. Rev. 77 (4): 313–348. doi:10.1021/cr60307a002.
 |