Physics:Aggregated diamond nanorod


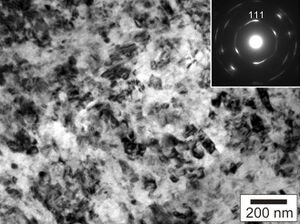
Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.
Discovery
Nanodiamond or hyperdiamond was produced by compression of graphite in 2003 by a group of researchers in Japan and in the same work, published in Nature, it was shown to be much harder than bulk diamond.[2] Later, it was also produced by compression of fullerene and confirmed to be the hardest and least compressible known material, with an isothermal bulk modulus of 491 gigapascals (GPa), while a conventional diamond has a modulus of 442–446 GPa; these results were inferred from X-ray diffraction data, which also indicated that ADNRs are 0.3% denser than regular diamond.[3] The same group later described ADNRs as "having a hardness and Young's modulus comparable to that of natural diamond, but with 'superior wear resistance'".[4]
Hardness
A <111> surface (normal to the largest diagonal of a cube) of pure diamond has a hardness value of 167±6 GPa when scratched with a nanodiamond tip, while the nanodiamond sample itself has a value of 310 GPa when tested with a nanodiamond tip. However, the test only works properly with a tip made of harder material than the sample being tested due to cracking. This means that the true value for nanodiamond is likely lower than 310 GPa.[5] Due to its hardness, a hyperdiamond could possibly exceed 10 on the Mohs scale of mineral hardness.
Synthesis
ADNRs (hyperdiamonds/nanodiamonds) are produced by compressing fullerite powder—a solid form of allotropic carbon fullerene—by either of two somewhat similar methods. One uses a diamond anvil cell and applied pressure ~37 GPa without heating the cell.[6] In another method, fullerite is compressed to lower pressures (2–20 GPa) and then heated to a temperature in the range of 300 to 2,500 K (27 to 2,227 °C).[7][8][9][10] Extreme hardness of what now appears likely to have been nanodiamonds was reported by researchers in the 1990s.[5][6] The material is a series of interconnected diamond nanorods, with diameters of between 5 and 20 nanometres and lengths of around 1 micrometre each.[citation needed]
Nanodiamond aggregates ca. 1 mm in size also form in nature, from graphite upon meteoritic impact, such as that of the Popigai impact structure in Siberia, Russia.[1]
See also
- Physics:Carbon nanotube – Allotropes of carbon with a cylindrical nanostructure
- Chemistry:Diamond – Form of carbon
- Chemistry:Lonsdaleite – Hexagonal lattice allotrope of carbon
- Physics:Mohs scale of mineral hardness – Qualitative scale characterizing scratch resistance
- Chemistry:Rhenium diboride
- Chemistry:Superhard material – Material with Vickers hardness exceeding 40 gigapascals
References
- ↑ 1.0 1.1 1.2 1.3 Ohfuji, Hiroaki; Irifune, Tetsuo; Litasov, Konstantin D.; Yamashita, Tomoharu; Isobe, Futoshi; Afanasiev, Valentin P.; Pokhilenko, Nikolai P. (2015). "Natural occurrence of pure nano-polycrystalline diamond from impact crater". Scientific Reports 5: 14702. doi:10.1038/srep14702. PMID 26424384. Bibcode: 2015NatSR...514702O.
- ↑ Irifune, Tetsuo; Kurio, Ayako; Sakamoto, Shizue; Inoue, Toru; Sumiya, Hitoshi (2003). "Materials: Ultrahard polycrystalline diamond from graphite". Nature 421 (6923): 599–600. doi:10.1038/421599b. PMID 12571587. Bibcode: 2003Natur.421..599I.
- ↑ Dubrovinskaia, Natalia; Dubrovinsky, Leonid; Crichton, Wilson; Langenhorst, Falko; Richter, Asta (2005). "Aggregated diamond nanorods, the densest and least compressible form of carbon". Applied Physics Letters 87 (8): 083106. doi:10.1063/1.2034101. Bibcode: 2005ApPhL..87h3106D. https://nbn-resolving.org/urn:nbn:de:kobv:526-opus4-5646.
- ↑ Dubrovinskaia, Natalia; Dub, Sergey; Dubrovinsky, Leonid (2006). "Superior Wear Resistance of Aggregated Diamond Nanorods". Nano Letters 6 (4): 824–6. doi:10.1021/nl0602084. PMID 16608291. Bibcode: 2006NanoL...6..824D.
- ↑ 5.0 5.1 Blank, V (1998). "Ultrahard and superhard phases of fullerite C60: Comparison with diamond on hardness and wear". Diamond and Related Materials 7 (2–5): 427–431. doi:10.1016/S0925-9635(97)00232-X. Bibcode: 1998DRM.....7..427B. Archived from the original on 2011-07-21. https://web.archive.org/web/20110721225258/http://nanoscan.info/wp-content/publications/article_03.pdf.
- ↑ 6.0 6.1 Blank, V; Popov, M; Buga, S; Davydov, V; Denisov, V; Ivlev, A; Marvin, B; Agafonov, V et al. (1994). "Is C60 fullerite harder than diamond?". Physics Letters A 188 (3): 281. doi:10.1016/0375-9601(94)90451-0. Bibcode: 1994PhLA..188..281B.
- ↑ Kozlov, M (1995). "Superhard form of carbon obtained from C60 at moderate pressure". Synthetic Metals 70 (1–3): 1411–1412. doi:10.1016/0379-6779(94)02900-J.
- ↑ Blank, V (1995). "Ultrahard and superhard carbon phases produced from C60 by heating at high pressure: structural and Raman studies". Physics Letters A 205 (2–3): 208–216. doi:10.1016/0375-9601(95)00564-J. Bibcode: 1995PhLA..205..208B.
- ↑ Szwarc, H; Davydov, V; Plotianskaya, S; Kashevarova, L; Agafonov, V; Ceolin, R (1996). "Chemical modifications of C under the influence of pressure and temperature: from cubic C to diamond". Synthetic Metals 77 (1–3): 265–272. doi:10.1016/0379-6779(96)80100-7.
- ↑ Blank, V (1996). "Phase transformations in solid C60 at high-pressure-high-temperature treatment and the structure of 3D polymerized fullerites". Physics Letters A 220 (1–3): 149–157. doi:10.1016/0375-9601(96)00483-5. Bibcode: 1996PhLA..220..149B.
External links
- The invention of aggregated diamond nanorods at Physorg.com
- Jeandron, Michelle (August 26, 2005). "Diamonds are not forever". Physics World. http://physicsworld.com/cws/article/news/22997.
 |

