Earth:Great Calcite Belt
File:Great Calcite Belt of the Southern Ocean.webm
The Great Calcite Belt (GCB) refers to a region of the ocean where there are high concentrations of calcite, a mineral form of calcium carbonate. The belt extends over a large area of the Southern Ocean surrounding Antarctica. The calcite in the Great Calcite Belt is formed by tiny marine organisms called coccolithophores, which build their shells out of calcium carbonate. When these organisms die, their shells sink to the bottom of the ocean, and over time, they accumulate to form a thick layer of calcite sediment.
The Great Calcite Belt occurs in areas of the Southern ocean where the calcite compensation depth (CCD) is relatively shallow, meaning that calcite minerals from the shells of marine organisms dissolve at a shallower depth in the water column. This results in a higher concentration of calcium carbonate sediments in the ocean floor, which can be observed in the form of white chalky sediments.
The Great Calcite Belt plays a significant role regulating the global carbon cycle. Calcite is a form of carbon that is removed from the atmosphere and stored in the ocean, which helps to reduce the amount of carbon dioxide in the atmosphere and mitigate the effects of climate change. Recent studies suggest the belt sequesters something between 15 and 30 million tonnes of carbon per year.[1][2]
Scientists have further interest in the calcite sediments in the belt, which contain valuable information about past climate, ocean currents, ocean chemistry, and marine ecosystems. For example, variations in the CCD depth over time can indicate changes in the amount of carbon dioxide in the atmosphere and the ocean's ability to absorb it. The belt is also home to a diverse range of contemporary marine life, including deep-sea corals and fish that are adapted to the unique conditions found in this part of the ocean. The Great Calcite Belt is a region of elevated summertime upper ocean calcite concentration derived from coccolithophores, despite the region being known for its diatom predominance. The overlap of two major phytoplankton groups, coccolithophores and diatoms, in the dynamic frontal systems characteristic of this region provides an ideal setting to study environmental influences on the distribution of different species within these taxonomic groups.[3]
Overview
The Great Calcite Belt can be defined as an elevated particulate inorganic carbon (PIC) feature occurring alongside seasonally elevated chlorophyll a in austral spring and summer in the Southern Ocean.[4] It plays an important role in climate fluctuations,[5][6] accounting for over 60% of the Southern Ocean area (30–60° S).[7] The region between 30° and 50° S has the highest uptake of anthropogenic carbon dioxide (CO2) alongside the North Atlantic and North Pacific oceans.[8] Knowledge of the impact of interacting environmental influences on phytoplankton distribution in the Southern Ocean is limited. For example, more understanding is needed of how light and iron availability or temperature and pH interact to control phytoplankton biogeography.[9][10][11] Hence, if model parameterizations are to improve to provide accurate predictions of biogeochemical change, a multivariate understanding of the full suite of environmental drivers is required.[12][3]
The Southern Ocean has often been considered as a microplankton-dominated (20–200 µm) system with phytoplankton blooms dominated by large diatoms and Phaeocystis sp.[13][14][15] However, since the identification of the Great Calcite Belt (GCB) as a consistent feature[4][16] and the recognition of picoplankton (< 2 µm) and nanoplankton (2–20 µm) importance in high-nutrient, low-chlorophyll (HNLC) waters,[17] the dynamics of small (bio)mineralizing plankton and their export need to be acknowledged. The two dominant biomineralizing phytoplankton groups in the GCB are coccolithophores and diatoms. Coccolithophores are generally found north of the polar front,[18] though Emiliania huxleyi has been observed as far south as 58° S in the Scotia Sea,[19] at 61° S across Drake Passage,[11] and at 65°S south of Australia.[20][3]
Diatoms are present throughout the GCB, with the polar front marking a strong divide between different size fractions.[21] North of the polar front, small diatom species, such as Pseudo-nitzschia spp. and Thalassiosira spp., tend to dominate numerically, whereas large diatoms with higher silicic acid requirements (e.g., Fragilariopsis kerguelensis) are generally more abundant south of the polar front.[21] High abundances of nanoplankton (coccolithophores, small diatoms, chrysophytes) have also been observed on the Patagonian Shelf [14] and in the Scotia Sea.[22] Currently, few studies incorporate small biomineralizing phytoplankton to species level.[21][13][14][22] Rather, the focus has often been on the larger and noncalcifying species in the Southern Ocean due to sample preservation issues (i.e., acidified Lugol’s solution dissolves calcite, and light microscopy restricts accurate identification to cells > 10 µm.[22] In the context of climate change and future ecosystem function, the distribution of biomineralizing phytoplankton is important to define when considering phytoplankton interactions with carbonate chemistry,[23][24] and ocean biogeochemistry.[25][26][27][3]
The Great Calcite Belt spans the major Southern Ocean circumpolar fronts: the Subantarctic front, the polar front, the Southern Antarctic Circumpolar Current front, and occasionally the southern boundary of the Antarctic Circumpolar Current.[28][29][30] The subtropical front (at approximately 10 °C) acts as the northern boundary of the GCB and is associated with a sharp increase in PIC southwards.[7] These fronts divide distinct environmental and biogeochemical zones, making the GCB an ideal study area to examine controls on phytoplankton communities in the open ocean.[15][9] A high PIC concentration observed in the GCB (1 µmol PIC L−1) compared to the global average (0.2 µmol PIC L−1) and significant quantities of detached E. huxleyi coccoliths (in concentrations > 20,000 coccoliths mL−1)[7] both characterize the GCB. The GCB is clearly observed in satellite imagery [4] spanning from the Patagonian Shelf [31][32] across the Atlantic, Indian, and Pacific oceans and completing Antarctic circumnavigation via the Drake Passage.[3]

Coccolithophores versus the diatom

The biogeography of Southern Ocean phytoplankton controls the local biogeochemistry and the export of macronutrients to lower latitudes and depth. Of particular relevance is the competitive interaction between coccolithophores and diatoms, with the former being prevalent along the Great Calcite Belt (40–60°S), while diatoms tend to dominate the regions south of 60°S, as illustrated in the diagram on the right.[33]
The ocean is changing at an unprecedented rate as a consequence of increasing anthropogenic CO2 emissions and related climate change. Changes in density stratification and nutrient supply, as well as ocean acidification, lead to changes in phytoplankton community composition and consequently ecosystem structure and function. Some of these changes are already observable today [34][35] and may have cascading effects on global biogeochemical cycles and oceanic carbon uptake.[36][37][38] Changes in Southern Ocean (SO) biogeography are especially critical due to the importance of the Southern Ocean in fuelling primary production at lower latitudes through the lateral export of nutrients [39] and in taking up anthropogenic CO2.[40] For the carbon cycle, the ratio of calcifying and noncalcifying phytoplankton is crucial due to the counteracting effects of calcification and photosynthesis on seawater pCO2, which ultimately controls CO2 exchange with the atmosphere, and the differing ballasting effect of calcite and silicic acid shells for organic carbon export.[33]

Calcifying coccolithophores and silicifying diatoms are globally ubiquitous phytoplankton functional groups.[41][42] Diatoms are a major contributor to global phytoplankton biomass [43] and annual net primary production.[44] In comparison, coccolithophores contribute less to biomass [43] and to global NPP.[45][46][47][48][33]
However, coccolithophores are the major phytoplanktonic calcifier.[49] thereby significantly impacting the global carbon cycle. Diatoms dominate the phytoplankton community in the Southern Ocean,[50][51][52] but coccolithophores have received increasing attention in recent years. Satellite imagery of particulate inorganic carbon (PIC, a proxy for coccolithophore abundance) revealed the "Great Calcite Belt",[53] an annually reoccurring circumpolar band of elevated PIC concentrations between 40 and 60°S. In situ observations confirmed coccolithophore abundances of up to 2.4×103 cells mL−1 in the Atlantic sector (blooms on the Patagonian Shelf), up to 3.8×102 cells mL−1 in the Indian sector,[16] and up to 5.4×102 cells mL−1 in the Pacific sector of the Southern Ocean [54] with Emiliania huxleyi being the dominant species.[16][55] However, the contribution of coccolithophores to total Southern Ocean phytoplankton biomass and NPP has not yet been assessed. Locally, elevated coccolithophore abundance in the GCB has been found to turn surface waters into a source of CO2 for the atmosphere,[16] emphasising the necessity to understand the controls on their abundance in the Southern Ocean in the context of the carbon cycle and climate change. While coccolithophores have been observed to have moved polewards in recent decades,[56][57][35] their response to the combined effects of future warming and ocean acidification is still subject to debate.[58][56][59][60][61] As their response will also crucially depend on future phytoplankton community composition and predator–prey interactions,[62] it is essential to assess the controls on their abundance in today's climate.[33]
Top-down and bottom-up approaches
Coccolithophore biomass is controlled by a combination of bottom-up (physical–biogeochemical environment) and top-down factors (predator–prey interactions), but the relative importance of the two has not yet been assessed for coccolithophores in the Southern Ocean. Bottom-up factors directly impact phytoplankton growth, and diatoms and coccolithophores are traditionally discriminated based on their differing requirements for nutrients, turbulence, and light. Based on this, Margalef's mandala predicts a seasonal succession from diatoms to coccolithophores as light levels increase and nutrient levels decline.[63] In situ studies assessing Southern Ocean coccolithophore biogeography have found coccolithophores under various environmental conditions,[16][64][65][55][51] thus suggesting a wide ecological niche, but all of the mentioned studies have almost exclusively focused on bottom-up controls.[33]
However, phytoplankton growth rates do not necessarily covary with biomass accumulation rates. Using satellite data from the North Atlantic, Behrenfeld stressed in 2014 the importance of simultaneously considering bottom-up and top-down factors when assessing seasonal phytoplankton biomass dynamics and the succession of different phytoplankton types owing to the spatially and temporally varying relative importance of the physical–biogeochemical and the biological environment.[66][33]
In the Southern Ocean, previous studies have shown zooplankton grazing to control total phytoplankton biomass,[67] phytoplankton community composition,[68] and ecosystem structure,[69][70] suggesting that top-down control might also be an important driver for the relative abundance of coccolithophores and diatoms. But the role of zooplankton grazing in current Earth system models is not well considered,[71][72] and the impact of different grazing formulations on phytoplankton biogeography and diversity is subject to ongoing research.[73][74][33]
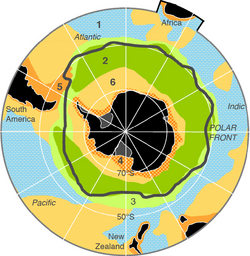
The diagram on the left shows the spatial distribution of different types of marine sediments in the Southern Ocean. The greenish area south of the Polar Front shows the extension of the subpolar opal belt where sediments have a significant portion of silicous plankton frustules. Sediments near Antarctica mainly consist of glacial debris in any grain size eroded and delivered by the Antarctic Ice.[75][76]
See also
References
- ↑ Anderson, Robert F.; Sachs, Julian P.; Fleisher, Martin Q.; Allen, Katherine A.; Yu, Jimin; Koutavas, Athanasios; Jaccard, Samuel L. (2019). "Deep‐Sea Oxygen Depletion and Ocean Carbon Sequestration During the Last Ice Age". Global Biogeochemical Cycles (American Geophysical Union (AGU)) 33 (3): 301–317. doi:10.1029/2018gb006049. ISSN 0886-6236. Bibcode: 2019GBioC..33..301A.
- ↑ Bain, Paul G.; Bongiorno, Renata (2019-10-23). "It's not too late to do the right thing: Moral motivations for climate change action". WIREs Climate Change (Wiley) 11 (1). doi:10.1002/wcc.615. ISSN 1757-7780.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Smith, Helen E. K.; Poulton, Alex J.; Garley, Rebecca; Hopkins, Jason; Lubelczyk, Laura C.; Drapeau, Dave T.; Rauschenberg, Sara; Twining, Ben S. et al. (2017). "The influence of environmental variability on the biogeography of coccolithophores and diatoms in the Great Calcite Belt". Biogeosciences 14 (21): 4905–4925. doi:10.5194/bg-14-4905-2017. Bibcode: 2017BGeo...14.4905S. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 4.0 4.1 4.2 Balch, W. M.; Gordon, Howard R.; Bowler, B. C.; Drapeau, D. T.; Booth, E. S. (2005). "Calcium carbonate measurements in the surface global ocean based on Moderate-Resolution Imaging Spectroradiometer data". Journal of Geophysical Research 110 (C7): C07001. doi:10.1029/2004JC002560. Bibcode: 2005JGRC..110.7001B.
- ↑ Sarmiento, Jorge L.; Hughes, Tertia M. C.; Stouffer, Ronald J.; Manabe, Syukuro (1998). "Simulated response of the ocean carbon cycle to anthropogenic climate warming". Nature 393 (6682): 245–249. doi:10.1038/30455. Bibcode: 1998Natur.393..245S.
- ↑ Sarmiento, J. L.; Slater, R.; Barber, R.; Bopp, L.; Doney, S. C.; Hirst, A. C.; Kleypas, J.; Matear, R. et al. (2004). "Response of ocean ecosystems to climate warming". Global Biogeochemical Cycles 18 (3): n/a. doi:10.1029/2003GB002134. Bibcode: 2004GBioC..18.3003S.
- ↑ 7.0 7.1 7.2 Balch, W. M.; Drapeau, D. T.; Bowler, B. C.; Lyczskowski, E.; Booth, E. S.; Alley, D. (2011). "The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Exchange Experiment: New evidence in support of the "Great Calcite Belt" hypothesis". Journal of Geophysical Research 116 (C4): C00F06. doi:10.1029/2011JC006941. Bibcode: 2011JGRC..116.0F06B.
- ↑ Sabine, C. L.; Feely, R. A.; Gruber, N.; Key, R. M.; Lee, K.; Bullister, J. L.; Wanninkhof, R.; Wong, C. S. et al. (2004). "The Oceanic Sink for Anthropogenic CO2". Science 305 (5682): 367–371. doi:10.1126/science.1097403. PMID 15256665. Bibcode: 2004Sci...305..367S. http://oceanrep.geomar.de/46251/1/1193.full.pdf.
- ↑ 9.0 9.1 Boyd, Philip W.; Strzepek, Robert; Fu, Feixue; Hutchins, David A. (2010). "Environmental control of open-ocean phytoplankton groups: Now and in the future". Limnology and Oceanography 55 (3): 1353–1376. doi:10.4319/lo.2010.55.3.1353. Bibcode: 2010LimOc..55.1353B.
- ↑ Boyd, P. W.; Arrigo, K. R.; Strzepek, R.; Van Dijken, G. L. (2012). "Mapping phytoplankton iron utilization: Insights into Southern Ocean supply mechanisms". Journal of Geophysical Research: Oceans 117 (C6): n/a. doi:10.1029/2011JC007726. Bibcode: 2012JGRC..117.6009B.
- ↑ 11.0 11.1 Charalampopoulou, Anastasia; Poulton, Alex J.; Bakker, Dorothee C. E.; Lucas, Mike I.; Stinchcombe, Mark C.; Tyrrell, Toby (2016). "Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean)". Biogeosciences 13 (21): 5917–5935. doi:10.5194/bg-13-5917-2016. Bibcode: 2016BGeo...13.5917C.
- ↑ Boyd, P.W.; Newton, P.P. (1999). "Does planktonic community structure determine downward particulate organic carbon flux in different oceanic provinces?". Deep Sea Research Part I: Oceanographic Research Papers 46 (1): 63–91. doi:10.1016/S0967-0637(98)00066-1. Bibcode: 1999DSRI...46...63B.
- ↑ 13.0 13.1 Bathmann, U.V.; Scharek, R.; Klaas, C.; Dubischar, C.D.; Smetacek, V. (1997). "Spring development of phytoplankton biomass and composition in major water masses of the Atlantic sector of the Southern Ocean". Deep-Sea Research Part II: Topical Studies in Oceanography 44 (1–2): 51–67. doi:10.1016/S0967-0645(96)00063-X. Bibcode: 1997DSRII..44...51B. https://epic.awi.de/id/eprint/193/1/Bat1997b.pdf.
- ↑ 14.0 14.1 14.2 Poulton, Alex J.; Mark Moore, C.; Seeyave, Sophie; Lucas, Mike I.; Fielding, Sophie; Ward, Peter (2007). "Phytoplankton community composition around the Crozet Plateau, with emphasis on diatoms and Phaeocystis". Deep-Sea Research Part II: Topical Studies in Oceanography 54 (18–20): 2085–2105. doi:10.1016/j.dsr2.2007.06.005. Bibcode: 2007DSRII..54.2085P.
- ↑ 15.0 15.1 Boyd, Philip W. (2002). "Environmental Factors Controlling Phytoplankton Processes in the Southern Ocean1". Journal of Phycology 38 (5): 844–861. doi:10.1046/j.1529-8817.2002.t01-1-01203.x.
- ↑ 16.0 16.1 16.2 16.3 16.4 Balch, William M.; Bates, Nicholas R.; Lam, Phoebe J.; Twining, Benjamin S.; Rosengard, Sarah Z.; Bowler, Bruce C.; Drapeau, Dave T.; Garley, Rebecca et al. (2016). "Factors regulating the Great Calcite Belt in the Southern Ocean and its biogeochemical significance". Global Biogeochemical Cycles 30 (8): 1124–1144. doi:10.1002/2016GB005414. Bibcode: 2016GBioC..30.1124B.
- ↑ Barber, R. T.; Hiscock, M. R. (2006). "A rising tide lifts all phytoplankton: Growth response of other phytoplankton taxa in diatom-dominated blooms". Global Biogeochemical Cycles 20 (4): n/a. doi:10.1029/2006GB002726. Bibcode: 2006GBioC..20.4S03B.
- ↑ Mohan, Rahul; Mergulhao, Lina P.; Guptha, M.V.S.; Rajakumar, A.; Thamban, M.; Anilkumar, N.; Sudhakar, M.; Ravindra, Rasik (2008). "Ecology of coccolithophores in the Indian sector of the Southern Ocean". Marine Micropaleontology 67 (1–2): 30–45. doi:10.1016/j.marmicro.2007.08.005. Bibcode: 2008MarMP..67...30M.
- ↑ Holligan, P.M.; Charalampopoulou, A.; Hutson, R. (2010). "Seasonal distributions of the coccolithophore, Emiliania huxleyi, and of particulate inorganic carbon in surface waters of the Scotia Sea". Journal of Marine Systems 82 (4): 195–205. doi:10.1016/j.jmarsys.2010.05.007. Bibcode: 2010JMS....82..195H.
- ↑ Cubillos, JC; Wright, SW; Nash, G.; De Salas, MF; Griffiths, B.; Tilbrook, B.; Poisson, A.; Hallegraeff, GM (2007). "Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: Changes in 2001 to 2006 compared to historical data". Marine Ecology Progress Series 348: 47–54. doi:10.3354/meps07058. Bibcode: 2007MEPS..348...47C.
- ↑ 21.0 21.1 21.2 Froneman, P.W.; McQuaid, C.D.; Perissinotto, R. (1995). "Biogeographic structure of the microphytoplankton assemblages of the south Atlantic and Southern Ocean during austral summer". Journal of Plankton Research 17 (9): 1791–1802. doi:10.1093/plankt/17.9.1791.
- ↑ 22.0 22.1 22.2 Hinz, D.J.; Poulton, A.J.; Nielsdóttir, M.C.; Steigenberger, S.; Korb, R.E.; Achterberg, E.P.; Bibby, T.S. (2012). "Comparative seasonal biogeography of mineralising nannoplankton in the Scotia Sea: Emiliania huxleyi, Fragilariopsis SPP. And Tetraparma pelagica". Deep-Sea Research Part II: Topical Studies in Oceanography 59-60: 57–66. doi:10.1016/j.dsr2.2011.09.002. Bibcode: 2012DSRII..59...57H.
- ↑ Langer, Gerald; Geisen, Markus; Baumann, Karl-Heinz; Kläs, Jessica; Riebesell, Ulf; Thoms, Silke; Young, Jeremy R. (2006). "Species-specific responses of calcifying algae to changing seawater carbonate chemistry". Geochemistry, Geophysics, Geosystems 7 (9): n/a. doi:10.1029/2005GC001227. Bibcode: 2006GGG.....7.9006L. https://epic.awi.de/id/eprint/14731/1/Lan2006e.pdf.
- ↑ Tortell, Philippe D.; Payne, Christopher D.; Li, Yingyu; Trimborn, Scarlett; Rost, Björn; Smith, Walker O.; Riesselman, Christina; Dunbar, Robert B. et al. (2008). "CO2sensitivity of Southern Ocean phytoplankton". Geophysical Research Letters 35 (4): L04605. doi:10.1029/2007GL032583. Bibcode: 2008GeoRL..35.4605T. https://digitalcommons.odu.edu/cgi/viewcontent.cgi?article=1089&context=oeas_fac_pubs.
- ↑ Baines, Stephen B.; Twining, Benjamin S.; Brzezinski, Mark A.; Nelson, David M.; Fisher, Nicholas S. (2010). "Causes and biogeochemical implications of regional differences in silicification of marine diatoms". Global Biogeochemical Cycles 24 (4): n/a. doi:10.1029/2010GB003856. Bibcode: 2010GBioC..24.4031B.
- ↑ Assmy, P.; Smetacek, V.; Montresor, M.; Klaas, C.; Henjes, J.; Strass, V. H.; Arrieta, J. M.; Bathmann, U. et al. (2013). "Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current". Proceedings of the National Academy of Sciences 110 (51): 20633–20638. doi:10.1073/pnas.1309345110. PMID 24248337. Bibcode: 2013PNAS..11020633A.
- ↑ Poulton, Alex J.; Painter, Stuart C.; Young, Jeremy R.; Bates, Nicholas R.; Bowler, Bruce; Drapeau, Dave; Lyczsckowski, Emily; Balch, William M. (2013). "The 2008Emiliania huxleyibloom along the Patagonian Shelf: Ecology, biogeochemistry, and cellular calcification". Global Biogeochemical Cycles 27 (4): 1023–1033. doi:10.1002/2013GB004641. Bibcode: 2013GBioC..27.1023P.
- ↑ Tsuchiya, Mizuki; Talley, Lynne D.; McCartney, Michael S. (1994). "Water-mass distributions in the western South Atlantic; A section from South Georgia Island (54S) northward across the equator". Journal of Marine Research 52: 55–81. doi:10.1357/0022240943076759.
- ↑ Orsi, Alejandro H.; Whitworth, Thomas; Nowlin, Worth D. (1995). "On the meridional extent and fronts of the Antarctic Circumpolar Current". Deep Sea Research Part I: Oceanographic Research Papers 42 (5): 641–673. doi:10.1016/0967-0637(95)00021-W. Bibcode: 1995DSRI...42..641O.
- ↑ Belkin, Igor M.; Gordon, Arnold L. (1996). "Southern Ocean fronts from the Greenwich meridian to Tasmania". Journal of Geophysical Research: Oceans 101 (C2): 3675–3696. doi:10.1029/95JC02750. Bibcode: 1996JGR...101.3675B.
- ↑ Signorini, Sergio R.; Garcia, Virginia M. T.; Piola, Alberto R.; Garcia, Carlos A. E.; Mata, Mauricio M.; McClain, Charles R. (2006). "Seasonal and interannual variability of calcite in the vicinity of the Patagonian shelf break (38°S–52°S)". Geophysical Research Letters 33 (16): L16610. doi:10.1029/2006GL026592. Bibcode: 2006GeoRL..3316610S.
- ↑ Painter, Stuart C.; Poulton, Alex J.; Allen, John T.; Pidcock, Rosalind; Balch, William M. (2010). "The COPAS'08 expedition to the Patagonian Shelf: Physical and environmental conditions during the 2008 coccolithophore bloom". Continental Shelf Research 30 (18): 1907–1923. doi:10.1016/j.csr.2010.08.013. Bibcode: 2010CSR....30.1907P.
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 Nissen, Cara; Vogt, Meike; Münnich, Matthias; Gruber, Nicolas; Haumann, F. Alexander (2018). "Factors controlling coccolithophore biogeography in the Southern Ocean". Biogeosciences 15 (22): 6997–7024. doi:10.5194/bg-15-6997-2018. Bibcode: 2018BGeo...15.6997N. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Cite error: Invalid
<ref>tag; name "Nissen2018" defined multiple times with different content - ↑ Soppa, Mariana; Völker, Christoph; Bracher, Astrid (2016). "Diatom Phenology in the Southern Ocean: Mean Patterns, Trends and the Role of Climate Oscillations". Remote Sensing 8 (5): 420. doi:10.3390/rs8050420. Bibcode: 2016RemS....8..420S.
- ↑ 35.0 35.1 Winter, Amos; Henderiks, Jorijntje; Beaufort, Luc; Rickaby, Rosalind E. M.; Brown, Christopher W. (2014). "Poleward expansion of the coccolithophore Emiliania huxleyi". Journal of Plankton Research 36 (2): 316–325. doi:10.1093/plankt/fbt110.
- ↑ Cermeno, P.; Dutkiewicz, S.; Harris, R. P.; Follows, M.; Schofield, O.; Falkowski, P. G. (2008). "The role of nutricline depth in regulating the ocean carbon cycle". Proceedings of the National Academy of Sciences 105 (51): 20344–20349. doi:10.1073/pnas.0811302106. PMID 19075222. Bibcode: 2008PNAS..10520344C.
- ↑ Freeman, Natalie M.; Lovenduski, Nicole S. (2015). "Decreased calcification in the Southern Ocean over the satellite record". Geophysical Research Letters 42 (6): 1834–1840. doi:10.1002/2014GL062769. Bibcode: 2015GeoRL..42.1834F. https://archimer.ifremer.fr/doc/00292/40371/.
- ↑ Laufkötter, Charlotte; Vogt, Meike; Gruber, Nicolas; Aumont, Olivier; Bopp, Laurent; Doney, Scott C.; Dunne, John P.; Hauck, Judith et al. (2016). "Projected decreases in future marine export production: The role of the carbon flux through the upper ocean ecosystem". Biogeosciences 13 (13): 4023–4047. doi:10.5194/bg-13-4023-2016. Bibcode: 2016BGeo...13.4023L.
- ↑ Sarmiento, J. L.; Gruber, N.; Brzezinski, M. A.; Dunne, J. P. (2004). "High-latitude controls of thermocline nutrients and low latitude biological productivity". Nature 427 (6969): 56–60. doi:10.1038/nature02127. PMID 14702082. Bibcode: 2004Natur.427...56S.
- ↑ Frölicher, Thomas L.; Sarmiento, Jorge L.; Paynter, David J.; Dunne, John P.; Krasting, John P.; Winton, Michael (2015). "Dominance of the Southern Ocean in Anthropogenic Carbon and Heat Uptake in CMIP5 Models". Journal of Climate 28 (2): 862–886. doi:10.1175/JCLI-D-14-00117.1. Bibcode: 2015JCli...28..862F.
- ↑ Leblanc, K.; Arístegui, J.; Armand, L.; Assmy, P.; Beker, B.; Bode, A.; Breton, E.; Cornet, V. et al. (2012). "A global diatom database – abundance, biovolume and biomass in the world ocean". Earth System Science Data 4 (1): 149–165. doi:10.5194/essd-4-149-2012. Bibcode: 2012ESSD....4..149L.
- ↑ O'Brien, C. J.; Peloquin, J. A.; Vogt, M.; Heinle, M.; Gruber, N.; Ajani, P.; Andruleit, H.; Arístegui, J. et al. (2013). "Global marine plankton functional type biomass distributions: Coccolithophores". Earth System Science Data 5 (2): 259–276. doi:10.5194/essd-5-259-2013. Bibcode: 2013ESSD....5..259O.
- ↑ 43.0 43.1 Buitenhuis, E. T.; Vogt, M.; Moriarty, R.; Bednaršek, N.; Doney, S. C.; Leblanc, K.; Le Quéré, C.; Luo, Y.-W. et al. (2013). "MAREDAT: Towards a world atlas of MARine Ecosystem DATa". Earth System Science Data 5 (2): 227–239. doi:10.5194/essd-5-227-2013. Bibcode: 2013ESSD....5..227B.
- ↑ Sarthou, Géraldine; Timmermans, Klaas R.; Blain, Stéphane; Tréguer, Paul (2005). "Growth physiology and fate of diatoms in the ocean: A review". Journal of Sea Research 53 (1–2): 25–42. doi:10.1016/j.seares.2004.01.007. Bibcode: 2005JSR....53...25S.
- ↑ Gregg, Watson W.; Casey, Nancy W. (2007). "Modeling coccolithophores in the global oceans". Deep-Sea Research Part II: Topical Studies in Oceanography 54 (5–7): 447–477. doi:10.1016/j.dsr2.2006.12.007. Bibcode: 2007DSRII..54..447G.
- ↑ Jin, X.; Gruber, N.; Dunne, J. P.; Sarmiento, J. L.; Armstrong, R. A. (2006). "Diagnosing the contribution of phytoplankton functional groups to the production and export of particulate organic carbon, CaCO3, and opal from global nutrient and alkalinity distributions". Global Biogeochemical Cycles 20 (2): n/a. doi:10.1029/2005GB002532. Bibcode: 2006GBioC..20.2015J.
- ↑ Moore, J. Keith; Doney, Scott C.; Lindsay, Keith (2004). "Upper ocean ecosystem dynamics and iron cycling in a global three-dimensional model". Global Biogeochemical Cycles 18 (4): n/a. doi:10.1029/2004GB002220. Bibcode: 2004GBioC..18.4028M.
- ↑ O'Brien, C. J. (2015) "Global-scale distributions of marine haptophyte phytoplankton", PhD thesis, ETH Zürich.
- ↑ Iglesias-Rodriguez, M. Debora; Armstrong, Robert; Feely, Richard; Hood, Raleigh; Kleypas, Joan; Milliman, John D.; Sabine, Christopher; Sarmiento, Jorge (2002). "Progress made in study of ocean's calcium carbonate budget". Eos, Transactions American Geophysical Union 83 (34): 365–375. doi:10.1029/2002EO000267.
- ↑ Swan, Chantal M.; Vogt, Meike; Gruber, Nicolas; Laufkoetter, Charlotte (2016). "A global seasonal surface ocean climatology of phytoplankton types based on CHEMTAX analysis of HPLC pigments". Deep Sea Research Part I: Oceanographic Research Papers 109: 137–156. doi:10.1016/j.dsr.2015.12.002. Bibcode: 2016DSRI..109..137S.
- ↑ 51.0 51.1 Trull, Thomas W.; Passmore, Abraham; Davies, Diana M.; Smit, Tim; Berry, Kate; Tilbrook, Bronte (2018). "Distribution of planktonic biogenic carbonate organisms in the Southern Ocean south of Australia: A baseline for ocean acidification impact assessment". Biogeosciences 15 (1): 31–49. doi:10.5194/bg-15-31-2018. Bibcode: 2018BGeo...15...31T.
- ↑ Wright, Simon W.; Van Den Enden, Rick L.; Pearce, Imojen; Davidson, Andrew T.; Scott, Fiona J.; Westwood, Karen J. (2010). "Phytoplankton community structure and stocks in the Southern Ocean (30–80°E) determined by CHEMTAX analysis of HPLC pigment signatures". Deep-Sea Research Part II: Topical Studies in Oceanography 57 (9–10): 758–778. doi:10.1016/j.dsr2.2009.06.015. Bibcode: 2010DSRII..57..758W.
- ↑ Balch, W. M.; Drapeau, D. T.; Bowler, B. C.; Lyczskowski, E.; Booth, E. S.; Alley, D. (2011). "The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Exchange Experiment: New evidence in support of the "Great Calcite Belt" hypothesis". Journal of Geophysical Research 116 (C4). doi:10.1029/2011JC006941. Bibcode: 2011JGRC..116.0F06B.
- ↑ Cubillos, JC; Wright, SW; Nash, G.; De Salas, MF; Griffiths, B.; Tilbrook, B.; Poisson, A.; Hallegraeff, GM (2007). "Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: Changes in 2001 to 2006 compared to historical data". Marine Ecology Progress Series 348: 47–54. doi:10.3354/meps07058. Bibcode: 2007MEPS..348...47C.
- ↑ 55.0 55.1 Saavedra-Pellitero, Mariem; Baumann, Karl-Heinz; Flores, José-Abel; Gersonde, Rainer (2014). "Biogeographic distribution of living coccolithophores in the Pacific sector of the Southern Ocean". Marine Micropaleontology 109: 1–20. doi:10.1016/j.marmicro.2014.03.003. Bibcode: 2014MarMP.109....1S.
- ↑ 56.0 56.1 Beaugrand, Gregory; McQuatters-Gollop, Abigail; Edwards, Martin; Goberville, Eric (2013). "Long-term responses of North Atlantic calcifying plankton to climate change". Nature Climate Change 3 (3): 263–267. doi:10.1038/nclimate1753. Bibcode: 2013NatCC...3..263B.
- ↑ Rivero-Calle, S.; Gnanadesikan, A.; Del Castillo, C. E.; Balch, W. M.; Guikema, S. D. (2015). "Multidecadal increase in North Atlantic coccolithophores and the potential role of rising CO2". Science 350 (6267): 1533–1537. doi:10.1126/science.aaa8026. PMID 26612836. Bibcode: 2015Sci...350.1533R.
- ↑ Beaufort, L.; Probert, I.; De Garidel-Thoron, T.; Bendif, E. M.; Ruiz-Pino, D.; Metzl, N.; Goyet, C.; Buchet, N. et al. (2011). "Sensitivity of coccolithophores to carbonate chemistry and ocean acidification". Nature 476 (7358): 80–83. doi:10.1038/nature10295. PMID 21814280.
- ↑ Iglesias-Rodriguez, M. D.; Halloran, P. R.; Rickaby, R. E. M.; Hall, I. R.; Colmenero-Hidalgo, E.; Gittins, J. R.; Green, D. R. H.; Tyrrell, T. et al. (2008). "Phytoplankton Calcification in a High-CO2 World". Science 320 (5874): 336–340. doi:10.1126/science.1154122. PMID 18420926. Bibcode: 2008Sci...320..336I.
- ↑ Riebesell, Ulf; Zondervan, Ingrid; Rost, Björn; Tortell, Philippe D.; Zeebe, Richard E.; Morel, François M. M. (2000). "Reduced calcification of marine plankton in response to increased atmospheric CO2". Nature 407 (6802): 364–367. doi:10.1038/35030078. PMID 11014189. Bibcode: 2000Natur.407..364R. https://epic.awi.de/id/eprint/3784/1/Rie2000a.pdf.
- ↑ Schlüter, Lothar; Lohbeck, Kai T.; Gutowska, Magdalena A.; Gröger, Joachim P.; Riebesell, Ulf; Reusch, Thorsten B. H. (2014). "Adaptation of a globally important coccolithophore to ocean warming and acidification". Nature Climate Change 4 (11): 1024–1030. doi:10.1038/nclimate2379. Bibcode: 2014NatCC...4.1024S.
- ↑ Dutkiewicz, Stephanie; Morris, J. Jeffrey; Follows, Michael J.; Scott, Jeffery; Levitan, Orly; Dyhrman, Sonya T.; Berman-Frank, Ilana (2015). "Impact of ocean acidification on the structure of future phytoplankton communities". Nature Climate Change 5 (11): 1002–1006. doi:10.1038/nclimate2722. Bibcode: 2015NatCC...5.1002D.
- ↑ Margalef, R. (1978) "Life-forms of phytoplankton as survival alternatives in an unstable environment", Oceanol. Acta, 1: 493–509.
- ↑ Charalampopoulou, Anastasia; Poulton, Alex J.; Bakker, Dorothee C. E.; Lucas, Mike I.; Stinchcombe, Mark C.; Tyrrell, Toby (2016). "Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean)". Biogeosciences 13 (21): 5917–5935. doi:10.5194/bg-13-5917-2016. Bibcode: 2016BGeo...13.5917C.
- ↑ Hinz, D.J.; Poulton, A.J.; Nielsdóttir, M.C.; Steigenberger, S.; Korb, R.E.; Achterberg, E.P.; Bibby, T.S. (2012). "Comparative seasonal biogeography of mineralising nannoplankton in the Scotia Sea: Emiliania huxleyi, Fragilariopsis SPP. And Tetraparma pelagica". Deep-Sea Research Part II: Topical Studies in Oceanography 59-60: 57–66. doi:10.1016/j.dsr2.2011.09.002. Bibcode: 2012DSRII..59...57H.
- ↑ Behrenfeld, Michael J. (2014). "Climate-mediated dance of the plankton". Nature Climate Change 4 (10): 880–887. doi:10.1038/nclimate2349. Bibcode: 2014NatCC...4..880B.
- ↑ Le Quéré, Corinne; Buitenhuis, Erik T.; Moriarty, Róisín; Alvain, Séverine; Aumont, Olivier; Bopp, Laurent; Chollet, Sophie; Enright, Clare et al. (2016). "Role of zooplankton dynamics for Southern Ocean phytoplankton biomass and global biogeochemical cycles". Biogeosciences 13 (14): 4111–4133. doi:10.5194/bg-13-4111-2016. Bibcode: 2016BGeo...13.4111L.
- ↑ Granĺi, Edna; Granéli, Wilhelm; Rabbani, Mohammed Mozzam; Daugbjerg, Niels; Fransz, George; Roudy, Janine Cuzin; Alder, Viviana A. (1993). "The influence of copepod and krill grazing on the species composition of phytoplankton communities from the Scotia Weddell sea". Polar Biology 13 (3): 201–213. doi:10.1007/BF00238930.
- ↑ De Baar, Hein J. W. et al. (2005). "Synthesis of iron fertilization experiments: From the Iron Age in the Age of Enlightenment". Journal of Geophysical Research 110 (C9). doi:10.1029/2004JC002601. Bibcode: 2005JGRC..110.9S16D.
- ↑ Smetacek, Victor; Assmy, Philipp; Henjes, Joachim (2004). "The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles". Antarctic Science 16 (4): 541–558. doi:10.1017/S0954102004002317. Bibcode: 2004AntSc..16..541S.
- ↑ Hashioka, T.; Vogt, M.; Yamanaka, Y.; Le Quéré, C.; Buitenhuis, E. T.; Aita, M. N.; Alvain, S.; Bopp, L. et al. (2013). "Phytoplankton competition during the spring bloom in four plankton functional type models". Biogeosciences 10 (11): 6833–6850. doi:10.5194/bg-10-6833-2013. Bibcode: 2013BGeo...10.6833H.
- ↑ Sailley, S.F.; Vogt, M.; Doney, S.C.; Aita, M.N.; Bopp, L.; Buitenhuis, E.T.; Hashioka, T.; Lima, I. et al. (2013). "Comparing food web structures and dynamics across a suite of global marine ecosystem models". Ecological Modelling 261-262: 43–57. doi:10.1016/j.ecolmodel.2013.04.006.
- ↑ Prowe, A.E. Friederike; Pahlow, Markus; Dutkiewicz, Stephanie; Follows, Michael; Oschlies, Andreas (2012). "Top-down control of marine phytoplankton diversity in a global ecosystem model". Progress in Oceanography 101 (1): 1–13. doi:10.1016/j.pocean.2011.11.016. Bibcode: 2012PrOce.101....1P.
- ↑ Vallina, S.M.; Ward, B.A.; Dutkiewicz, S.; Follows, M.J. (2014). "Maximal feeding with active prey-switching: A kill-the-winner functional response and its effect on global diversity and biogeography". Progress in Oceanography 120: 93–109. doi:10.1016/j.pocean.2013.08.001. Bibcode: 2014PrOce.120...93V.
- ↑ Diekmann, B. (2007). Sedimentary patterns in the late Quaternary Southern Ocean, Deep-Sea Res. II, 54, 2350-2366, doi:10.1016/j.dsr2.2007.07.025.
- ↑ Grobe, H., Diekmann, B., Hillenbrand, C.-D.(2009). The memory of the Polar Oceans, In: Hempel, G. (ed) Biology of Polar Oceans, hdl:10013/epic.33599.d001, pdf 0.4 MB.
 |