Chemistry:Phosphatidylinositol (3,4,5)-trisphosphate
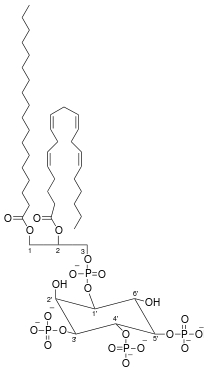
| |
| Names | |
|---|---|
| Other names
PI(3,4,5)P3, PtdIns(3,4,5)P3
| |
| Identifiers | |
| ChEBI | |
| KEGG | |
| Properties | |
| C47H86O22P4 | |
| Molar mass | 1126.46 g/mol, neutral with fatty acid composition - 18:0, 20:4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
Discovery
In 1988, Lewis C. Cantley published a paper describing the discovery of a novel type of phosphoinositide kinase with the unprecedented ability to phosphorylate the 3' position of the inositol ring resulting in the formation of phosphatidylinositol-3-phosphate (PI3P).[1] Working independently, Alexis Traynor-Kaplan and coworkers published a paper demonstrating that a novel lipid, phosphatidylinositol 3,4,5 trisphosphate (PIP3) occurs naturally in human neutrophils with levels that increased rapidly following physiologic stimulation with chemotactic peptide.[2] Subsequent studies demonstrated that in vivo the enzyme originally identified by Cantley's group prefers PtdIns(4,5)P2 as a substrate, producing the product PIP3.[3]
Function
PIP3 functions to activate downstream signaling components, the most notable one being the protein kinase Akt, which activates downstream anabolic signaling pathways required for cell growth and survival.[4]
PtdIns(3,4,5)P3 is dephosphorylated by the phosphatase PTEN on the 3 position, generating PI(4,5)P2, and by SHIPs (SH2-containing inositol phosphatase) on the 5' position of the inositol ring, producing PI(3,4)P2.[5]
The PH domain in a number of proteins binds to PtdIns(3,4,5)P3. Such proteins include Akt/PKB,[6] PDPK1,[7] Btk1, and ARNO.[8]
Roles in the nervous system
PIP3 plays a critical role outside the cytosol, notably at the postsynaptic terminal of hippocampal cells. Here, PIP3 has been implicated in regulating synaptic strengthening and AMPA expression, contributing to long-term potentiation. Moreover, PIP3 suppression disrupts normal AMPA expression on the neuron membrane and instead leads to the accumulation of AMPA on dendritic spines, commonly associated with synaptic depression.[9]
PIP3 interacts with proteins to mediate synaptic plasticity. Of these proteins, Phldb2 has been shown to interact with PIP3 to induce and maintain long-term potentiation. In the absence of such an interaction, memory consolidation is impaired.[10]
References
- ↑ "Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate". Nature 332 (6165): 644–6. April 1988. doi:10.1038/332644a0. PMID 2833705. Bibcode: 1988Natur.332..644W.
- ↑ "An inositol tetrakisphosphate-containing phospholipid in activated neutrophils". Nature 334 (6180): 353–6. July 1988. doi:10.1038/334353a0. PMID 3393226. Bibcode: 1988Natur.334..353T.
- ↑ "PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells". Cell 57 (1): 167–75. April 1989. doi:10.1016/0092-8674(89)90182-7. PMID 2467744.
- ↑ Ma, Qi; Zhu, Chongzhuo; Zhang, Weilin; Ta, Na; Zhang, Rong; Liu, Lei; Feng, Du; Cheng, Heping et al. (January 2019). "Mitochondrial PIP3-binding protein FUNDC2 supports platelet survival via AKT signaling pathway". Cell Death and Differentiation 26 (2): 321–331. doi:10.1038/s41418-018-0121-8. ISSN 1476-5403. PMID 29786068.
- ↑ Qi, Yanmei; Liu, Jie; Chao, Joshua; Greer, Peter A.; Li, Shaohua (2020-09-07). "PTEN dephosphorylates Abi1 to promote epithelial morphogenesis". The Journal of Cell Biology 219 (9). doi:10.1083/jcb.201910041. ISSN 1540-8140. PMID 32673396.
- ↑ Eramo, Matthew J.; Mitchell, Christina A. (February 2016). "Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases". Biochemical Society Transactions 44 (1): 240–252. doi:10.1042/BST20150214. ISSN 1470-8752. PMID 26862211. https://research.monash.edu/files/226576341/3592704_oa.pdf.
- ↑ Gagliardi, Paolo Armando; Puliafito, Alberto; Primo, Luca (February 2018). "PDK1: At the crossroad of cancer signaling pathways". Seminars in Cancer Biology 48: 27–35. doi:10.1016/j.semcancer.2017.04.014. ISSN 1096-3650. PMID 28473254. https://pubmed.ncbi.nlm.nih.gov/28473254/.
- ↑ Venkateswarlu, Kanamarlapudi; Oatey, Paru B.; Tavaré, Jeremy M.; Cullen, Peter J. (April 1998). "Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase" (in en). Current Biology 8 (8): 463–466. doi:10.1016/s0960-9822(98)70181-2. ISSN 0960-9822. PMID 9550703.
- ↑ Arendt, Kristin L.; Royo, María; Fernández-Monreal, Mónica; Knafo, Shira; Petrok, Cortney N.; Martens, Jeffrey R.; Esteban, José A. (January 2010). "PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane". Nature Neuroscience 13 (1): 36–44. doi:10.1038/nn.2462. ISSN 1546-1726. PMID 20010819.
- ↑ Xie, Min-Jue; Ishikawa, Yasuyuki; Yagi, Hideshi; Iguchi, Tokuichi; Oka, Yuichiro; Kuroda, Kazuki; Iwata, Keiko; Kiyonari, Hiroshi et al. (13 March 2019). "PIP3-Phldb2 is crucial for LTP regulating synaptic NMDA and AMPA receptor density and PSD95 turnover". Scientific Reports 9 (1): 4305. doi:10.1038/s41598-019-40838-6. ISSN 2045-2322. PMID 30867511. Bibcode: 2019NatSR...9.4305X.
 |

