Biology:Bruton's tyrosine kinase
 Generic protein structure example |
Bruton's tyrosine kinase (abbreviated Btk or BTK), also known as tyrosine-protein kinase BTK, is a tyrosine kinase that is encoded by the BTK gene in humans. BTK plays a crucial role in B cell development.
Structure
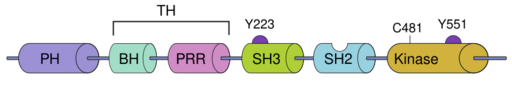
BTK contains five different protein interaction domains. These domains include an amino terminal pleckstrin homology (PH) domain, a proline-rich TEC homology (TH) domain, SRC homology (SH) domains SH2 and SH3, as well as a kinase domain with enzymatic activity.[1]
Function
BTK plays a crucial role in B cell development as it is required for transmitting signals from the pre-B cell receptor that forms after successful immunoglobulin heavy chain rearrangement.[2] It also has a role in mast cell activation through the high-affinity IgE receptor.[3]
Btk contains a PH domain that binds phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 binding induces Btk to phosphorylate phospholipase C, which in turn hydrolyzes PIP2, a phosphatidylinositol, into two second messengers, inositol triphosphate (IP3) and diacylglycerol (DAG), which then go on to modulate the activity of downstream proteins during B-cell signalling.[citation needed]
Clinical significance
Mutations in the BTK gene are implicated in the primary immunodeficiency disease X-linked agammaglobulinemia (Bruton's agammaglobulinemia); sometimes abbreviated to XLA and selective IgM deficiency.[4] Patients with XLA have normal pre-B cell populations in their bone marrow but these cells fail to mature and enter the circulation. The Btk gene is located on the X chromosome (Xq21.3-q22).[5] At least 400 mutations of the BTK gene have been identified. Of these, at least 212 are considered to be disease-causing mutations.[6]
BTK inhibitors
Approved drugs that inhibit BTK:
- Ibrutinib (Imbruvica), a selective Bruton's tyrosine kinase inhibitor.
- Acalabrutinib (Calquence), approved in October 2017[7] for relapsed mantle cell lymphoma.
- Zanubrutinib (Brukinsa) for mantle cell lymphoma, chronic lymphocytic leukemia, or small lymphocytic lymphoma.[8] It can be taken by mouth.[9][10]
- Tirabrutinib (Velexbru), approved in March 2020, in Japan, for the treatment of recurrent or refractory primary central nervous system lymphoma.[11]
- Pirtobrutinib (Jaypirca), a reversible (non-covalent) inhibitor of BTK, for mantle cell lymphoma.[12][13]
Various drugs that inhibit BTK are in clinical trials:[14]
- Phase 3:
- Acalabrutinib, for relapsed chronic lymphocytic leukemia (CLL), 95% overall remission reported.
- Evobrutinib for multiple sclerosis.[15][16][17]
- Tolebrutinib, for multiple sclerosis.[18][19]
- Remibrutinib, for multiple sclerosis.[20]
- Fenebrutinib (RG7845) for multiple sclerosis.[21][22]
- Phase 2:
- Phase 1:
Discovery
Bruton's tyrosine kinase was discovered in 1993 and is named for Ogden Bruton, who first described XLA in 1952.[5]
Interactions
Bruton's tyrosine kinase has been shown to interact with:
- ARID3A[29]
- BLNK (SLP-65),[30][31]
- CAV1,[32]
- GNAQ,[33]
- GTF2I,[34][35][36]
- PLCG2,[30][37]
- PRKD1,[38] and
- SH3BP5.[39][40]
References
- ↑ "Role of Bruton's tyrosine kinase in B cells and malignancies". Molecular Cancer 17 (1): 57. February 2018. doi:10.1186/s12943-018-0779-z. PMID 29455639.
- ↑ Kuby Immunology (7th ed.). New York: W.H. Freeman. 2013. p. 93. ISBN 978-14641-3784-6.
- ↑ "Signalling through the high-affinity IgE receptor Fc epsilonRI". Nature 402 (6760 Suppl): B24–B30. November 1999. doi:10.1038/35037021. PMID 10586892.
- ↑ "Hypomorphic Mutations in the BCR Signalosome Lead to Selective Immunoglobulin M Deficiency and Impaired B-cell Homeostasis". Frontiers in Immunology 9: 2984. 18 August 2018. doi:10.3389/fimmu.2018.02984. PMID 30619340.
- ↑ 5.0 5.1 X-Linked Agammaglobulinemia Patient and Family Handbook for The Primary Immune Diseases. Third Edition. 2001. Published by the Immune Deficiency Foundation.
- ↑ "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports 9 (1): 18577. December 2019. doi:10.1038/s41598-019-54976-4. PMID 31819097. Bibcode: 2019NatSR...918577S.
- ↑ "FDA approves new treatment for adults with mantle cell lymphoma". 24 March 2020. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm583076.htm.
- ↑ "FDA approves therapy to treat patients with relapsed and refractory mantle cell lymphoma supported by clinical trial results showing high response rate of tumor shrinkage". U.S. Food and Drug Administration (FDA) (Press release). 14 November 2019. Retrieved 15 November 2019.
- ↑ BeiGene Announces Initiation of a Combination Trial of the BTK Inhibitor BGB-3111 with the PD-1 Antibody BGB-A317. June 2016
- ↑ "FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma". U.S. Food and Drug Administration (FDA). 19 January 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma.
- ↑ "Tirabrutinib: First Approval". Drugs 80 (8): 835–840. June 2020. doi:10.1007/s40265-020-01318-8. PMID 32382949.
- ↑ "FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma". U.S. Food and Drug Administration (FDA). 27 January 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pirtobrutinib-relapsed-or-refractory-mantle-cell-lymphoma.
- ↑ "Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study". Lancet 397 (10277): 892–901. March 2021. doi:10.1016/S0140-6736(21)00224-5. PMID 33676628.
- ↑ "BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies". Journal of Hematology & Oncology 15 (1): 138. October 2022. doi:10.1186/s13045-022-01353-w. PMID 36183125.
- ↑ "Placebo-Controlled Trial of an Oral BTK Inhibitor in Multiple Sclerosis". The New England Journal of Medicine 380 (25): 2406–2417. June 2019. doi:10.1056/NEJMoa1901981. PMID 31075187.
- ↑ Clinical trial number NCT02975349 for "A Study of Efficacy and Safety of M2951 in Subjects With Relapsing Multiple Sclerosis" at ClinicalTrials.gov
- ↑ Clinical trial number NCT04032171 for "A Phase III, Multicenter, Randomized, Parallel Group, Double Blind, Double Dummy, Active Controlled Study of Evobrutinib Compared With an Interferon Beta 1a (Avonex®), in Participants With RMS to Evaluate Efficacy and Safety " at ClinicalTrials.gov
- ↑ "BTK blockers make headway in multiple sclerosis". Nature Biotechnology 39 (1): 3–5. January 2021. doi:10.1038/s41587-020-00790-7. PMID 33432223.
- ↑ Clinical trial number NCT04742400 for "A Phase 2 Clinical Trial of Tolebrutinib, a Brain-penetrant Bruton s Tyrosine Kinase Inhibitor, for the Modulation of Chronically Inflamed White Matter Lesions in Multiple Sclerosis" at ClinicalTrials.gov
- ↑ "Remibrutinib for Multiple Sclerosis". BioNews, Inc.. 21 June 2022. https://multiplesclerosisnewstoday.com/remibrutinib-multiple-sclerosis/.
- ↑ "Genentech: Our Pipeline" (in en-us). https://www.gene.com/medical-professionals/pipeline.
- ↑ Hoffmann-La Roche (2023-05-30). A Phase III Multicenter, Randomized, Double-Blind, Double-Dummy, Parallel-Group Study to Evaluate the Efficacy and Safety of Fenebrutinib Compared With Ocrelizumab in Adult Patients With Primary Progressive Multiple Sclerosis.. https://clinicaltrials.gov/ct2/show/NCT04544449.
- ↑ Clinical trial number NCT03978520 for "A Study to Investigate the Safety and Efficacy of ABBV-105 and Upadacitinib Given Alone or in Combination in Participants With Moderately to Severely Active Systemic Lupus Erythematosus - Full Text View - ClinicalTrials.gov" at ClinicalTrials.gov
- ↑ "Genentech: Our Pipeline". https://www.gene.com/medical-professionals/pipeline.
- ↑ Clinical trial number NCT01659255 for "ONO-4059 Phase I Dose-escalation Study to Investigate the Safety and Tolerability of ONO-4059 Given as Monotherapy in Patients With Relapsed/Refractory Non-Hodgkin's Lymphoma and/or Chronic Lymphocytic Leukaemi" at ClinicalTrials.gov
- ↑ "Novel BTK, PI3K Inhibitors on Horizon for Relapsed CLL. March 2016". http://global.onclive.com/conference-coverage/hematology-2016/novel-btk-pi3k-inhibitors-on-horizon-for-relapsed-cll.
- ↑ Clinical trial number NCT01351935 for "Escalating Dose Study in Subjects With Relapsed or Refractory B Cell Non-Hodgkin Lymphoma, Chronic Lymphocytic Leukemia, and Waldenstrom's Macroglobulinemia" at ClinicalTrials.gov
- ↑ "Lilly inks a $690M deal to get its hands on an autoimmune drug" (in en). FierceBiotech. 19 March 2015. http://www.fiercebiotech.com/r-d/lilly-inks-a-690m-deal-to-get-its-hands-on-an-autoimmune-drug.
- ↑ "The transcription factor, Bright, is not expressed in all human B lymphocyte subpopulations". Cellular Immunology 228 (1): 42–53. March 2004. doi:10.1016/j.cellimm.2004.03.004. PMID 15203319.
- ↑ 30.0 30.1 "Cbl-b positively regulates Btk-mediated activation of phospholipase C-gamma2 in B cells". The Journal of Experimental Medicine 196 (1): 51–63. July 2002. doi:10.1084/jem.20020068. PMID 12093870.
- ↑ "Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling". Blood 94 (7): 2357–2364. October 1999. doi:10.1182/blood.V94.7.2357.419k40_2357_2364. PMID 10498607.
- ↑ "Functional interaction of caveolin-1 with Bruton's tyrosine kinase and Bmx". The Journal of Biological Chemistry 277 (11): 9351–9357. March 2002. doi:10.1074/jbc.M108537200. PMID 11751885.
- ↑ "Identification of the binding site for Gqalpha on its effector Bruton's tyrosine kinase". Proceedings of the National Academy of Sciences of the United States of America 95 (21): 12197–12201. October 1998. doi:10.1073/pnas.95.21.12197. PMID 9770463. Bibcode: 1998PNAS...9512197M.
- ↑ "Mechanism of Bruton's tyrosine kinase-mediated recruitment and regulation of TFII-I". The Journal of Biological Chemistry 279 (8): 7147–7158. February 2004. doi:10.1074/jbc.M303724200. PMID 14623887.
- ↑ "Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase". Molecular and Cellular Biology 19 (7): 5014–5024. July 1999. doi:10.1128/mcb.19.7.5014. PMID 10373551.
- ↑ "BAP-135, a target for Bruton's tyrosine kinase in response to B cell receptor engagement". Proceedings of the National Academy of Sciences of the United States of America 94 (2): 604–609. January 1997. doi:10.1073/pnas.94.2.604. PMID 9012831. Bibcode: 1997PNAS...94..604Y.
- ↑ "Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex". Immunity 13 (2): 243–253. August 2000. doi:10.1016/s1074-7613(00)00024-8. PMID 10981967.
- ↑ "Bruton's tyrosine kinase (Btk) associates with protein kinase C mu". FEBS Letters 461 (1–2): 68–72. November 1999. doi:10.1016/S0014-5793(99)01424-6. PMID 10561498.
- ↑ "Identification and characterization of a novel SH3-domain binding protein, Sab, which preferentially associates with Bruton's tyrosine kinase (BtK)". Biochemical and Biophysical Research Communications 245 (2): 337–343. April 1998. doi:10.1006/bbrc.1998.8420. PMID 9571151.
- ↑ "Bruton's tyrosine kinase activity is negatively regulated by Sab, the Btk-SH3 domain-binding protein". Proceedings of the National Academy of Sciences of the United States of America 96 (11): 6341–6346. May 1999. doi:10.1073/pnas.96.11.6341. PMID 10339589. Bibcode: 1999PNAS...96.6341Y.
Further reading
- "Advances in X-linked immunodeficiency diseases". Current Opinion in Pediatrics 5 (6): 684–691. December 1993. doi:10.1097/00008480-199312000-00008. PMID 7907259.
- "Bruton's tyrosine kinase (BTK) as a dual-function regulator of apoptosis". Biochemical Pharmacology 56 (6): 683–691. September 1998. doi:10.1016/S0006-2952(98)00122-1. PMID 9751072.
- "B cell signaling. Introduction". International Reviews of Immunology 20 (6): 675–678. 2001. doi:10.3109/08830180109045584. PMID 11913944.
- "Novel aspects of hypogammaglobulinemic states". The Israel Medical Association Journal 4 (4): 294–297. April 2002. PMID 12001708.
- "Branches of the B cell antigen receptor pathway are directed by protein conduits Bam32 and Carma1". Immunity 19 (5): 637–640. November 2003. doi:10.1016/S1074-7613(03)00303-0. PMID 14614850.
- "Btk-dependent regulation of phosphoinositide synthesis". Biochemical Society Transactions 32 (Pt 2): 326–329. April 2004. doi:10.1042/BST0320326. PMID 15046600.
- "Involvement of SLP-65 and Btk in tumor suppression and malignant transformation of pre-B cells". Seminars in Immunology 18 (1): 67–76. February 2006. doi:10.1016/j.smim.2005.10.002. PMID 16300960.
External links
- GeneReviews/NCBI/NIH/UW entry on X-Linked or Brunton's Agammaglobulinemia
- Bruton's+tyrosine+kinase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human BTK genome location and BTK gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: Q06187 (Tyrosine-protein kinase BTK) at the PDBe-KB.
 |