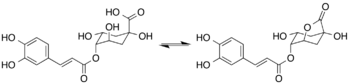
Chemistry:4-Caffeoyl-1,5-quinide
From HandWiki
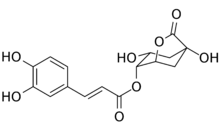
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S,3R,4R,5R)-1,3-Dihydroxy-7-oxo-6-oxabicyclo[3.2.1]octan-4-yl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | |
| Other names
4-Caffeoylquinic-1,5-lactone; 4-CQL
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H16O8 | |
| Molar mass | 336.296 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Caffeoyl-1,5-quinide (4-caffeoylquinic-1,5-lactone or 4-CQL) is found in roasted coffee beans. It is formed by lactonization of 4-O-caffeoylquinic acid during the roasting process.[1]
It is reported to possess opioid antagonist properties in mice.[2]
References
- ↑ Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Blackwell Publishing Ltd. 2006. p. 275. https://archive.org/details/plantsecondaryme00croz_308.
- ↑ de Paulis, Tomas; Commers, Patricia; Farah, Adriana; Zhao, Jiali; McDonald, Michael P.; Galici, Ruggero; Martin, Peter R. (2004). "4-Caffeoyl-1,5-quinide in roasted coffee inhibits [3Hnaloxone binding and reverses anti-nociceptive effects of morphine in mice"]. Psychopharmacology 176 (2): 146–153. doi:10.1007/s00213-004-1876-9. PMID 15088081. http://vanderbilt.edu/ics/Files/QUINIDE_opioids.pdf. Retrieved 2013-05-29.
 |