Chemistry:Mitiperstat
From HandWiki
Short description: Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
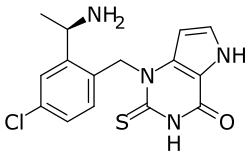
| Formula | C15H15ClN4OS |
| Molar mass | 334.82 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mitiperstat (AZD4831) is an irreversible inhibitor of myeloperoxidase and experimental drug in development for heart failure with preserved ejection fraction.[1][2][3][4][5][6][7] It is being developed by AstraZeneca.[8]
References
- ↑ Michaëlsson, Erik; Lund, Lars H.; Hage, Camilla; Shah, Sanjiv J.; Voors, Adriaan A.; Saraste, Antti; Redfors, Björn; Grove, Erik L. et al. (July 2023). "Myeloperoxidase Inhibition Reverses Biomarker Profiles Associated With Clinical Outcomes in HFpEF". JACC: Heart Failure 11 (7): 775–787. doi:10.1016/j.jchf.2023.03.002. PMID 37140510.
- ↑ Lam, Carolyn S.P.; Lund, Lars H.; Shah, Sanjiv J.; Voors, Adriaan A.; Erlinge, David; Saraste, Antti; Pirazzi, Carlo; Grove, Erik L. et al. (April 2023). "Myeloperoxidase Inhibition in Heart Failure With Preserved or Mildly Reduced Ejection Fraction: SATELLITE Trial Results". Journal of Cardiac Failure. doi:10.1016/j.cardfail.2023.04.003. PMID 37072105.
- ↑ Bhattacharya, Chandrali; Sandinge, Ann-Sofie; Bragg, Ryan A.; Heijer, Maria; Yan, Jingjing; Andersson, Linda C.; Jurva, Ulrik; Pelay-Gimeno, Marta et al. (April 2023). "Application of Accelerator Mass Spectrometry to Characterize the Mass Balance Recovery and Disposition of AZD4831, a Novel Myeloperoxidase Inhibitor, following Administration of an Oral Radiolabeled Microtracer Dose in Humans". Drug Metabolism and Disposition 51 (4): 451–463. doi:10.1124/dmd.122.001100. PMID 36639243.
- ↑ Jurva, Ulrik; Weidolf, Lars; Sandinge, Ann-Sofie; Leandersson, Carina; Ekdahl, Anja; Li, Xue-Qing; Antonsson, Thomas; Sundell, Johan et al. (April 2023). "Biotransformation of the Novel Myeloperoxidase Inhibitor AZD4831 in Preclinical Species and Humans". Drug Metabolism and Disposition 51 (4): 464–479. doi:10.1124/dmd.122.001099. PMID 36653117.
- ↑ Nelander, Karin; Lagerstrom‐Fermer, Maria; Amilon, Carl; Michaëlsson, Erik; Heijer, Maria; Kjaer, Magnus; Russell, Muir; Han, David et al. (May 2021). "Early Clinical Experience With AZD4831, A Novel Myeloperoxidase Inhibitor, Developed for Patients With Heart Failure With Preserved Ejection Fraction". Clinical and Translational Science 14 (3): 812–819. doi:10.1111/cts.12859. PMID 32770730.
- ↑ Gan, Li‐Ming; Lagerström‐Fermér, Maria; Ericsson, Hans; Nelander, Karin; Lindstedt, Eva‐Lotte; Michaëlsson, Erik; Kjaer, Magnus; Heijer, Maria et al. (April 2019). "Safety, tolerability, pharmacokinetics and effect on serum uric acid of the myeloperoxidase inhibitor AZD4831 in a randomized, placebo‐controlled, phase I study in healthy volunteers". British Journal of Clinical Pharmacology 85 (4): 762–770. doi:10.1111/bcp.13855. PMID 30618054.
- ↑ Inghardt, Tord; Antonsson, Thomas; Ericsson, Cecilia; Hovdal, Daniel; Johannesson, Petra; Johansson, Carina; Jurva, Ulrik; Kajanus, Johan et al. (8 September 2022). "Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction". Journal of Medicinal Chemistry 65 (17): 11485–11496. doi:10.1021/acs.jmedchem.1c02141. PMID 36005476.
- ↑ "AstraZeneca's investigational MPO inhibitor AZD4831 shows promise in patients with heart failure and a preserved ejection fraction". July 2021. https://www.astrazeneca.com/media-centre/medical-releases/astrazenecas-investigational-mpo-inhibitor-azd4831-shows-promise-in-patients-with-heart-failure-and-a-preserved-ejection-fraction.html.
 |

