Chemistry:Sulfilimine
In chemistry, a sulfilimine (or sulfimide) is a type of chemical compound containing a sulfur-to-nitrogen bond which is often represented as a double bond (S=N). In fact, a double bond violates the octet rule, and the bond may be considered a single bond with a formal charge of +1 on the sulfur and a formal charge of −1 on the nitrogen. The parent compound is sulfilimine H
2S=NH, which is mainly of theoretical interest.
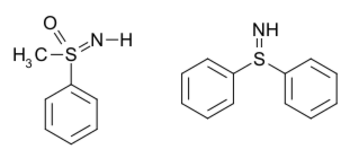
Examples include S,S-diphenylsulfilimine[2] and sulfoximines [Category] such as methylphenylsulfoximine:[3] In the case of a sulfoximine, the bonds can be considered single bonds, with formal charges of −1 on both the oxygen and the nitrogen, and a formal charge of +2 on the sulfur.
Preparation
Most sulfilimines are N-substituted with electron-withdrawing groups. These compounds are typically prepared by oxidation of thioethers with electrophilic amine reagents, such as chloramine-T in the presence of a base:[4]
- [math]\displaystyle{ \mathbf{:}\ce{R2S + ClNHTs -\gt R2S=NTs + HCl} }[/math]
An alternative route involves reactions of electrophilic sulfur compounds with amines. The imidosulfonium reagents provide a source of "Me
2S2+", which are attacked by amines.
Sulfilimine bonds in proteins
Sulfilimine bonds stabilize collagen IV strands found in the extracellular matrix[5] and arose at least 500 mya.[6] These bonds covalently connect hydroxylysine and methionine residues of adjacent polypeptide strands to form a larger collagen trimer.
References
- ↑ The preparation and structure of novel sulfimide systems; X-ray crystal structures of 1,4-(PhS{NH})2C6H4(and dihydrate), 1,2-(PhS{NH})(PhS)C6H4·H2O and of [Ph2SNH] and its hydrate Mark R. J. Elsegood, Kathryn E. Holmes, Paul F. Kelly, Jonathan Parr and Julia M. Stonehouse New J. Chem., 2002, 26, 202 - 206. doi:10.1039/b103502a
- ↑ S,S-Diphenylsulfilimine – Sigma-Aldrich
- ↑ (R)-(−)-S-Methyl-S-phenylsulfoximine – Sigma-Aldrich
- ↑ Gilchrist, T. L.; Moody, C. J., "The chemistry of sulfilimines", Chem. Rev. 1977, 77, 409-435. doi:10.1021/cr60307a005
- ↑ "A sulfilimine bond identified in collagen IV.". Science 325 (5945): 1230–1234. September 4, 2009. doi:10.1126/science.1176811. PMID 19729652. Bibcode: 2009Sci...325.1230V.
- ↑ A unique covalent bond in basement membrane is a primordial innovation for tissue evolution PNAS
 |