Chemistry:1,4,7-Trimethyl-1,4,7-triazacyclononane
From HandWiki

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4,7-Trimethyl-1,4,7-triazonane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H21N3 | |
| Molar mass | 171.288 g·mol−1 |
| Appearance | Colorless oil |
| Boiling point | 207.8 °C (406.0 °F; 480.9 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
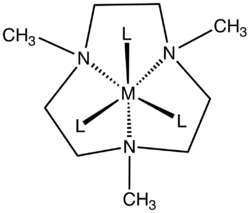
1,4,7-Trimethyl-1,4,7-triazacyclononane is the aza-crown ether with the formula (CH2CH2NCH3)3. This colorless liquid is the N-methylated derivative of triazacyclononane (TACN), a face-capping tridentate ligand that is popular in coordination chemistry.
Although TACN is known for forming 2:1 "sandwich" complexes with many metal ions,[1] corresponding 2:1 complexes of Me3TACN are only known for Ag+,[2] Na+,[3] and K+.[4] This effect is mainly due to the greater bulk of Me3TACN, which requires ions with a larger ionic radius to accommodate two ligands.
Several related derivatives have been prepared with diverse substituents on nitrogen.[5][6]
References
- ↑ Weighardt, Karl (1988). "1,4,7-Triazacyclononane and N,N',N"-Trimethyl-1,4,7-triazacyclononane - Two Versatile Macrocycles for the Synthesis of Monomeric and Oligomeric Metal Complexes". Pure and Applied Chemistry 60 (4): 509–16. doi:10.1351/pac198860040509.
- ↑ Stockheim, Claudia; Wieghardt, Karl; Nuber, Bernhard; Weiss, Johannes; Flöurke, Ulrich; Haupt, Hans-Jürgen (1991). "Co-ordination chemistry of 1,4,7-triazacyclononane (L) and its N-methylated derivative (L′) with silver( I ) and mercury( II ). The crystal structures of [AgL′ 2 PF 6 and [AgL′(SCN)]"] (in en). J. Chem. Soc., Dalton Trans. (6): 1487–1490. doi:10.1039/DT9910001487. ISSN 0300-9246. http://xlink.rsc.org/?DOI=DT9910001487.
- ↑ Everett, Matthew; Jolleys, Andrew; Levason, William; Pugh, David; Reid, Gillian (2014). "Unexpected neutral aza-macrocycle complexes of sodium" (in en). Chemical Communications 50 (44): 5843–6. doi:10.1039/c4cc01407c. ISSN 1359-7345. PMID 24759888. http://xlink.rsc.org/?DOI=c4cc01407c.
- ↑ Dyke, John; Levason, William; Light, Mark E.; Pugh, David; Reid, Gillian; Bhakhoa, Hanusha; Ramasami, Ponnadurai; Rhyman, Lydia (2015). "Aza-macrocyclic complexes of the Group 1 cations – synthesis, structures and density functional theory study" (in en). Dalton Trans. 44 (31): 13853–13866. doi:10.1039/C5DT01865J. ISSN 1477-9226. PMID 26115444. http://xlink.rsc.org/?DOI=C5DT01865J.
- ↑ Jason A. Halfen; William B. Tolman (1998). "C 2 -Symmetric 1,4-Diisopropyl-7- R -1,4,7-Triazacyclononanes". Inorganic Syntheses. 32. pp. 75–81. doi:10.1002/9780470132630.ch12. ISBN 9780470132630.
- ↑ Baker, M. V.; Brown, D. H.; Skelton, B. W.; White, A. H. (2002). "An Investigation into Alkenyl-Functionalized 1,4,7-Triazacyclononanes: Synthesis, Metal Complexation, and Attempted Olefin Metathesis". Australian Journal of Chemistry 55 (10): 655. doi:10.1071/CH02063.
 |