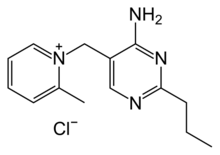
Chemistry:Amprolium
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-[(4-Amino-2-propylpyrimidin-5-yl)methyl]-2-methylpyridin-1-ium chloride | |
| Other names
1-[(4-Amino-2-propyl-5-pyrimidinyl)methyl]-2-picolinium chloride, Amprovine, Amprolium, Amprol, Anticoccid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| MeSH | Amprolium |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H19N4+ · Cl− | |
| Molar mass | 278.780 g·mol−1 |
| Pharmacology | |
| 1=ATCvet code} | QP51BX02 (WHO) QP51BX52 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Amprolium is the organic compound sold as a coccidiostat used in poultry. It has many International Nonproprietary Names.[1][clarification needed]
Uses in coccidiosis treatment in poultry
The drug is a thiamine analogue and blocks the thiamine transporter of Eimeria species. By blocking thiamine uptake it prevents carbohydrate synthesis.[citation needed]
Despite only moderate efficacy it is well favoured due to few resistance issues and is commonly used in the United States in conjunction with sulfonamides prophylactically in chickens and cattle as a coccidiostat.[citation needed]
Synthesis
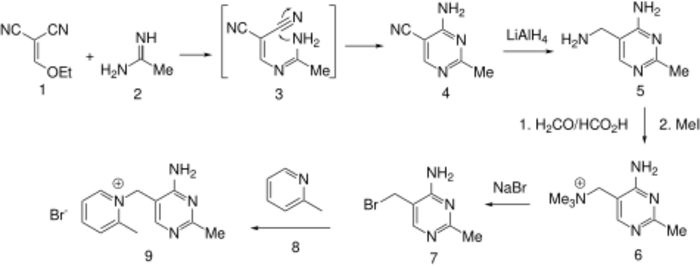
Condensation of ethoxymethylenemalononitrile (1) with acetamidine (2) affords the substituted pyrimidine (4). The reaction may well involve conjugate addition of the amidine nitrogen to the malononitrile followed by loss of ethoxide (3); addition of the remaining amidine nitrogen to one of the nitriles will then lead to the pyrimidine (4). Reduction of the nitrile gives the corresponding aminomethyl compound (5). Exhaustive methylation of the amine followed by displacement of the activated quaternary nitrogen by bromide ion affords the key intermediate (7). Displacement of the halogen by α-picoline gives amprolium.
References
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- ↑ Grewe, R. Z. Physiol. Chem. (1936).
- ↑ "Study on the Synthesis Process of Amprolium Hydrochloride - Master's thesis - Dissertation". Archived from the original on 2015-04-02. https://web.archive.org/web/20150402131023/http://www.dissertationtopic.net/doc/1133386. Retrieved 2015-03-30.
 |

