Chemistry:Aflavinine
From HandWiki
| |
| Names | |
|---|---|
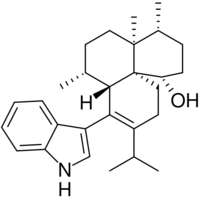
| IUPAC name
(1S,4R,4aS,7R,7aS,11aS)-8-(1H-Indol-3-yl)-4,4a,7-trimethyl-9-propan-2-yl-1,2,3,4,5,6,7,7a,10,11-decahydrobenzo[i]naphthalen-1-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H39NO | |
| Molar mass | 405.626 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Aflavinine is an anti-insectan chemical compound produced by some plants and fungi.[1][2][3]
References
- ↑ Gallagher, Rex T.; McCabe, Terrence; Hirotsu, Ken; Clardy, Jon; Nicholson, Judith; Wilson, Benjamin J. (January 1980). "Aflavinine, a novel indole-mevalonate metabolite from tremorgen-producing species". Tetrahedron Letters 21 (3): 243–246. doi:10.1016/s0040-4039(00)71179-8. ISSN 0040-4039.
- ↑ Gloer, James B.; TePaske, Mark R.; Sima, John S.; Wicklow, Donald T.; Dowd, Patrick F. (November 1988). "Antiinsectan aflavinine derivatives from the sclerotia of Aspergillus flavus" (in EN). The Journal of Organic Chemistry 53 (23): 5457–5460. doi:10.1021/jo00258a011. ISSN 0022-3263.
- ↑ Wang, H. J.; Gloer, J. B.; Wicklow, D. T.; Dowd, P. F. (Dec 1995). "Aflavinines and other antiinsectan metabolites from the ascostromata of Eupenicillium crustaceum and related species". Applied and Environmental Microbiology 61 (12): 4429–4435. doi:10.1128/aem.61.12.4429-4435.1995. ISSN 0099-2240. PMID 8534106.
 |

