Chemistry:Potassium trithiocarbonate
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CK2S3 | |
| Molar mass | 186.39 g·mol−1 |
| Appearance | solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
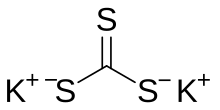
Potassium trithiocarbonate is the inorganic compound with the formula K
2CS
3. It consists of two potassium cations and the planar trithiocarbonate dianion. It is a white solid, although impure samples often appear brown. It is prepared by the reaction of potassium sulfide or potassium hydrosulfide with carbon disulfide. Potassium trithiocarbonate reacts with alkylating agents to give trithiocarbonate esters:[1]
- K
2CS
3 + 2 RX → (RS)
2CS + 2 KX (X = halide)
References
- ↑ R. E. Strube (1959). "Trithiocarbodiglycolic Acid". Organic Syntheses 39: 77. doi:10.15227/orgsyn.039.0077.
 |

