Chemistry:Cetostearyl alcohol
From HandWiki
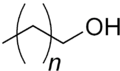 n = ~14-16
| |
| Names | |
|---|---|
| Other names
Cetearyl alcohol; Cetylstearyl alcohol; Cetyl/stearyl alcohol
| |
| Identifiers | |
| ChemSpider |
|
| EC Number |
|
| UNII | |
| Properties | |
| CH3(CH2)nCH2OH; n=variable, typically 14-16 | |
| Melting point | 48 to 56 °C (118 to 133 °F; 321 to 329 K)[1] |
| Hazards | |
| Safety data sheet | makingcosmetics.com |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H413 | |
| P281, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cetostearyl alcohol, cetearyl alcohol or cetylstearyl alcohol[1] is a mixture of fatty alcohols, consisting predominantly of cetyl (16 C) and stearyl alcohols (18 C) and is classified as a fatty alcohol. It is used as an emulsion stabilizer, opacifying agent, and foam boosting surfactant, as well as an aqueous and nonaqueous viscosity-increasing agent. It imparts an emollient feel to the skin and can be used in water-in-oil emulsions, oil-in-water emulsions, and anhydrous formulations. It is commonly used in hair conditioners and other hair products.[2]
5% cetyl stearyl alcohol in petrolatum is cytotoxic per the MTT assay.
References
 |


