Chemistry:Zinc picolinate
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H8N2O4Zn | |
| Molar mass | 309.59 g·mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H315, H319, H335, H410 | |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
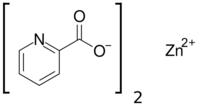
Zinc picolinate (or ZnPic) is the zinc salt of picolinic acid which has the molecular formula Zn(C6H4O2N)2.[1]
Zinc picolinate has been used as a dietary zinc supplement.[2][3][4]
References
- ↑ "The crystal structure of zinc picolinate tetrahydrate". Acta Chemica Scandinavica 23: 3011–3022. 1969. doi:10.3891/acta.chem.scand.23-3011. http://actachemscand.org/pdf/acta_vol_23_p3011-3022.pdf.
- ↑ "Zinc: health effects and research priorities for the 1990s". Environmental Health Perspectives 102 (Suppl 2): 5–46. June 1994. doi:10.1289/ehp.941025. PMID 7925188.
- ↑ "Double-blind, placebo-controlled trial of zinc picolinate for taste disorders". Acta Oto-Laryngologica. Supplementum 122 (546): 129–33. 2002. doi:10.1080/00016480260046517. PMID 12132610.
- ↑ "Comparative absorption of zinc picolinate, zinc citrate and zinc gluconate in humans". Agents and Actions 21 (1–2): 223–8. June 1987. doi:10.1007/BF01974946. PMID 3630857.
 |

