Chemistry:Bromal hydrate
From HandWiki
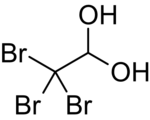
| |
| Names | |
|---|---|
| IUPAC name
2,2,2-Tribromo-1,1-ethanediol
| |
| Other names
tribromoethylidene glycol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H3Br3O2 | |
| Molar mass | 298.756 g·mol−1 |
| Appearance | white crystalline solid with bromal-like smell and taste[1] |
| Melting point | 53.5 °C (128.3 °F; 326.6 K) |
| Soluble | |
| Solubility | Soluble in ethanol, Diethyl ether, chloroform and Glycerin |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Bromal hydrate is an organobromine compound with the chemical formula C
2H
3Br
3O
2. It is the bromine analogue of chloral hydrate. Bromal hydrate forms when bromal is reacted with water. It decomposes to bromal and water upon distillation.[1] It has hypnotic and analgesic properties but acts like a stimulant at lower doses.[3] Bromal hydrate is more physiologically active than its chlorine analogue, chloral hydrate.[4] Its direct effect on the heart muscles is stronger than that of chloral hydrate.[3] Its analgesic effects were attributed to the proposed metabolism to bromoform.[5]
It was also tried as a medication for epilepsy, but was found ineffective.[1]
References
- ↑ 1.0 1.1 1.2 BROMAL – BROMAL HYDRATE, Stillé, A., Maisch, J. M. (1884). The National Dispensatory: Containing the Natural History, Chemistry, Pharmacy, Actions, and Uses of Medicine. Including Those Recognized in the Pharmacopoeias of the United States, Great Britain, and Germany, with Numerous References to the French Codex. H.C. Lea.
- ↑ "Bromal hydrate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/68181#section=Safety-and-Hazards.
- ↑ 3.0 3.1 Bromal Hydrate (page 53), Cerna, D. (1894). Notes on the newer remedies. USA: W.B. Saunders.
- ↑ BENNETT, J. H. (1875). Researches into the Antagonism of Medicines; being the report of the Edinburgh Committee of the British Medical Association ... Reprint from the British Medical Journal. J.&A. Churchill.
- ↑ Bromal Hydrate, E. Steinauer (1871)
 |

