Chemistry:Trifluoromethyl cation
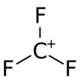 The structure of the cation.
| |
| Names | |
|---|---|
| Systematic IUPAC name
Trifluoromethylcarbenium | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| CF3+ | |
| Molar mass | 69.0054 |
| reacts | |
| Structure | |
| Trigonal planar | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The trifluoromethyl cation is a molecular cation with a formula of CF+3. It is a carbocation due to its positively charged carbon atom. It is part of the family of carbenium ions, with three fluorine atoms as substituents in place of its hydrogen atoms.[1]
Stability
Compared to methenium (the simplest carbenium ion), trifluoromethyl cation is more stable due to the presence of fluorine atoms. The fluorine atoms have lone pairs of electrons overlapping with the carbon atom. These electrons stabilize the positive charge of the central carbon atom, stabilizing the molecule as a whole. The overlap is effective due to the size of fluorine's p orbital in the molecule.[2]
Synthesis
While electron-donating fluorine lone pairs are present, it does not exist as its own.[clarification needed] The production of a CF+3 cation has been described as "extremely hard". The first relevant reagent, a diaryl(trifluoromethyl) sulfonium salt (Ar2S+CF3SbF−6) was developed in 1984 by reaction of an aryltrifluoromethyl sulfoxide 1 with SF+3SbF−6 followed by reaction with an electron-rich arene. Now the reaction of the source of the cation[clarification needed] usually uses 5-(trifluoromethyl)dibenzothiophenium tetrafluoroborate as the reagent.[3]
References
- ↑ "Trifluoromethyl cation" (in en). https://webbook.nist.gov/cgi/cbook.cgi?ID=C18851768&Mask=8.
- ↑ Wade, L. G. (2013). Organic Chemistry. Glenview, IL: Pearson Education, Inc.. pp. 162–163. ISBN 978-0-321-76841-4.
- ↑ Barata‐Vallejo, Sebastián; Lantaño, Beatriz; Postigo, Al (2014). "Recent Advances in Trifluoromethylation Reactions with Electrophilic Trifluoromethylating Reagents" (in en). Chemistry – A European Journal 20 (51): 16806–16829. doi:10.1002/chem.201404005. ISSN 1521-3765. PMID 25335765. https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201404005.


