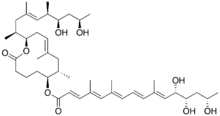
Chemistry:Mycolactone
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6S,7S,9E,12R)-12-[(2S,4E,6R,7R,9R)-7,9-Dihydroxy-4,6-dimethyldec-4-en-2-yl]-7,9-dimethyl-2-oxo-1-oxacyclododec-9-en-6-yl (2E,4E,6E,8E,10E,12S,13S,15S)-12,13,15-trihydroxy-4,6,10-trimethylhexadeca-2,4,6,8,10-pentaenoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | Mycolactone |
PubChem CID
|
|
| |
| |
| Properties | |
| C44H70O9 | |
| Molar mass | 743.021 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mycolactone is a polyketide-derived macrolide produced and secreted by a group of very closely related pathogenic mycobacteria species including M. ulcerans, M. liflandii (an unofficial designation), M. pseudoshottsii, and some strains of M. marinum. These mycobacteria are collectively referred to as mycolactone-producing mycobacteria or MPM.[1][2]
In humans, mycolactone is the toxin responsible for Buruli ulcers, doing so by damaging tissues and inhibiting the immune response.[3]
Variants
Five distinct, naturally occurring mycolactone structural variants have been described so far:
- Mycolactone A/B (M. ulcerans from Africa, Malaysia, Japan[4]
- Mycolactone C (M. ulcerans from Australia)[5]
- Mycolactone D (M. ulcerans from China)
- Mycolactone E (M. liflandii from Sub-Saharan Africa)[6][7]
- Mycolactone F (M. pseudoshottsii and M. marinum from around the world)[8][9]
Biosynthesis
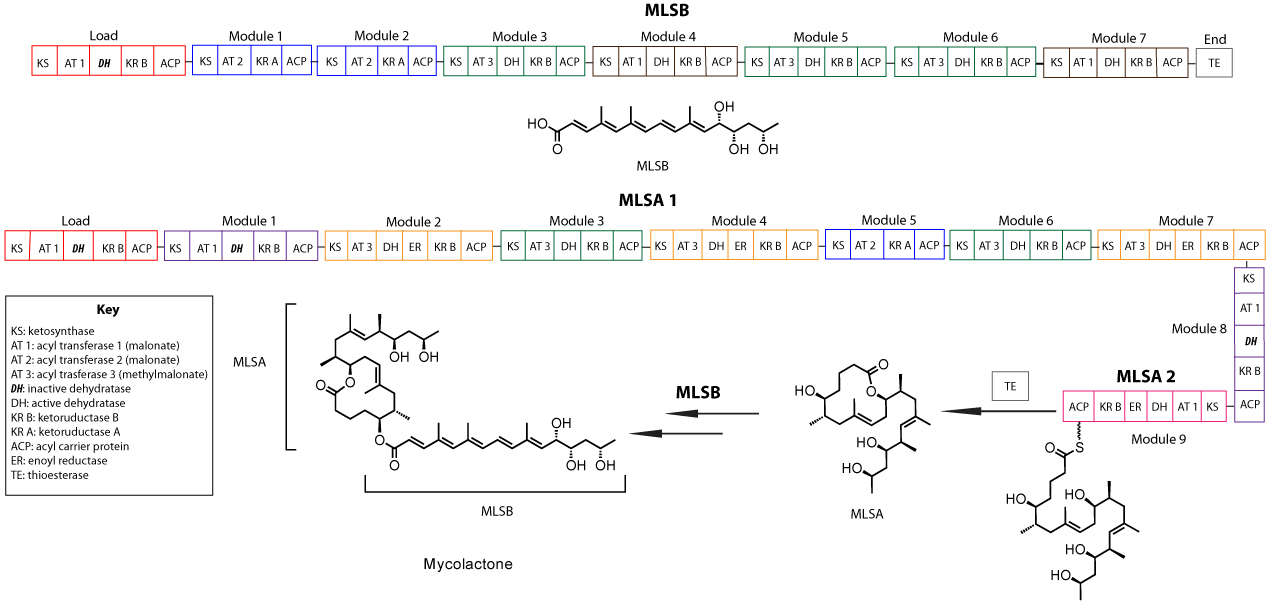
Mycolactone consists of a 12-membered macrolide core with an ester-linked polyketide chain. Three plasmid-encoded polyketide synthase (PKS) enzymes are responsible for its production: MLSA 1 and MLSA 2 which generate the core, and MLSB is responsible for the synthesis of the polyketide chain. As shown in Figure 1, MLSB (1.2 MDa) contains seven consecutive extension modules and MLSA 1 (1.8 MDa) consists of eight. The remaining PKS enzyme, MLSA 2, contains the ninth module of MLSA.[10] The C-terminal domains of both MLSA2 and MLSB includes a thioesterase (TE) that was thought to catalyze the formation of the mycolactone core but appears inactive.[11] Each module consists of either malonyl-CoA or methylmalonyl-CoA Acyltransferase (AT) that allows for chain extension, a ketosynthase (KS), which catalyzes chain elongation, and an Acyl carrier protein (ACP) where the growing polyketide chain is attached. Modules may also consist of any of the following modifying domains: a dehydratase (DH), an enoyl reductase (ER) and one of two types of ketoreductase (KR) domains. Type A and B KRs refer to the two directions of ketoreduction that are correlated with specific amino acids in the active site. Four of the DH domains are predicted to be inactive based on a point mutation found in the active site sequence.[10][12]
Figure 1. Domain Organization of Mycolactone.[10]
References
- ↑ "Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor.". J. Bacteriol. 189 (5): 2021–29. March 2007. doi:10.1128/JB.01442-06. PMID 17172337.
- ↑ Käser M; Hauser J; Small P; Pluschke G. (September 2009). "Large sequence polymorphisms unveil the phylogenetic relationship of environmental and pathogenic mycobacteria related to "Mycobacterium ulcerans".". Appl Environ Microbiol 75 (17): 5667–75. doi:10.1128/AEM.00446-09. PMID 19592526. Bibcode: 2009ApEnM..75.5667K.
- ↑ "Buruli ulcer disease". Fact sheets. WHO. March 2007. https://www.who.int/mediacentre/factsheets/fs199/en/.
- ↑ "Deciphering the genetic basis for polyketide variation among mycobacteria producing mycolactones.". BMC Genomics 9: 462. October 2008. doi:10.1186/1471-2164-9-462. PMID 18840298.
- ↑ Mve-Obiang, Armand; Lee, Richard E.; Portaels, Françoise; Small, P. L. C. (February 2003). "Heterogeneity of Mycolactones Produced by Clinical Isolates of Mycobacterium ulcerans : Implications for Virulence". Infection and Immunity 71 (2): 774–783. doi:10.1128/IAI.71.2.774-783.2003. PMID 12540557.
- ↑ "A newly discovered mycobacterial pathogen isolated from laboratory colonies of Xenopus species with lethal infections produces a novel form of mycolactone, the Mycobacterium ulcerans macrolide toxin.". Infect. Immun. 73 (6): 3307–12. June 2005. doi:10.1128/IAI.73.6.3307-3312.2005. PMID 15908356.
- ↑ Hong H; Stinear T; Skelton P; Spencer JB; Leadlay PF. (Sep 2005). "Structure elucidation of a novel family of mycolactone toxins from the frog pathogen Mycobacterium sp. MU128FXT by mass spectrometry.". Chem Commun 34 (34): 4306–8. doi:10.1039/b506835e. PMID 16113730.
- ↑ "Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F.". Infect. Immun. 74 (11): 6037–45. November 2006. doi:10.1128/IAI.00970-06. PMID 16923788.
- ↑ Stragier P; Hermans K; Stinear T; Portaels F. (September 2008). "First report of a mycolactone-producing Mycobacterium infection in fish agriculture in Belgium.". FEMS Microbiol. Lett. 286 (1): 93–5. doi:10.1111/j.1574-6968.2008.01264.x. PMID 18631185.
- ↑ 10.0 10.1 10.2 "Giant plasmid-encoded polyketide synthases produce the macrolide toxin of "Mycobacterium ulcerans".". PNAS 101 (6): 1345–9. February 2004. doi:10.1073/pnas.0305877101. PMID 14736915.
- ↑ Meier, Jordan L.; Barrows-Yano, Tiffany; Foley, Timothy L.; Wike, Candice L.; Burkart, Michael D. (2008). "The unusual macrocycle forming thioesterase of mycolactone". Molecular BioSystems 4 (6): 663–671. doi:10.1039/b801397g. PMID 18493665.
- ↑ Bali, Shilpa; Weissman, Kira J. (4 December 2006). "Ketoreduction in Mycolactone Biosynthesis: Insight into Substrate Specificity and Stereocontrol from Studies of Discrete Ketoreductase Domains in vitro". ChemBioChem 7 (12): 1935–1942. doi:10.1002/cbic.200600285. PMID 17031885.
 |